CATEGORY: Decontamination

Jul 18, 2024
Reprocessing Stories: Resolving Ergonomic Challenges in Henry Ford’s GI Department
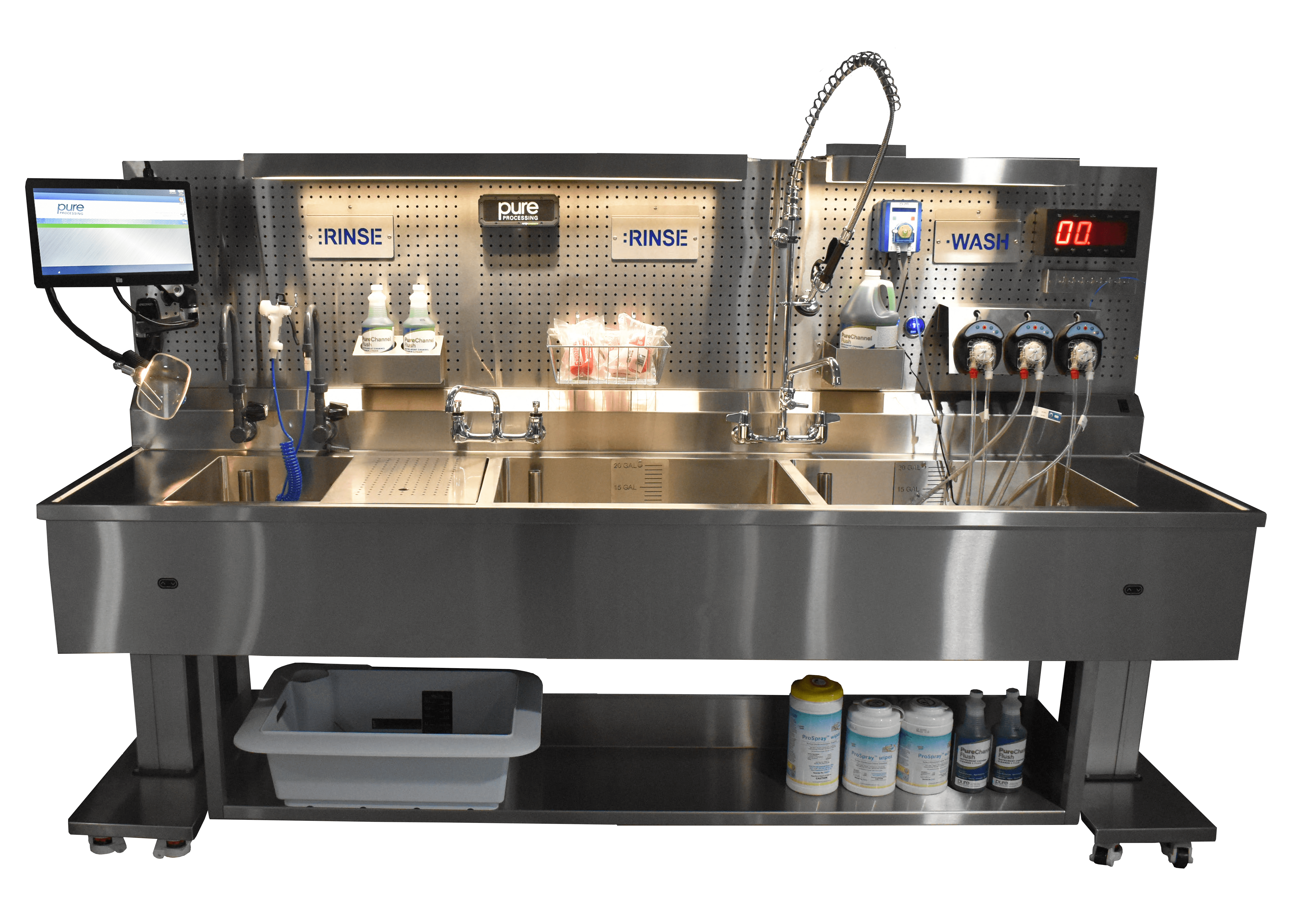
Over the past 37 years, Kate Jahnke has served her patients and staff at the Henry Ford Health System across multiple departments, 20 in GI. Jahnke’s journey into the position was somewhat serendipitous. She transitioned into GI after an initial role in the ER and since 2020, she’s been working at Henry Ford Health System, Columbus, first as a contingent and then full-time. When her long-time supervisor left, she was encouraged to step into the role. Jahnke is also accompanied by longtime team member April Helton, who has worked alongside Jahnke as a medical assistant for 16 years. Henry Ford & Jahnke’s Super Star Team Jahnke’s department operates in a freestanding ambulatory site; a three-story building that is part of the Henry Ford Health System. This system includes their main campus downtown and several ambulatory sites across the area, as well as a larger department in West Bloomfield Hospital. Her site operates five days a week, reprocessing approximately 150 procedures a week or around 30-38 procedures daily. Jahnke is proud to say her team is staffed with the highest quality endoscopy techs. As Jahnke states: “Our loyalties I think are rare… and the camaraderie is rare. Anytime we get a big group of people, especially women, we support each other in many aspects of our [personal] and our work lives.” This mutual support creates an enjoyable and fulfilling workplace. The Henry Ford team takes pride in the quality of their care, and their patients can sense that positive environment. It’s part of the secret that inspires Jahnke and her team. Ergonomic Concerns in Decontamination While Jahnke and Helton had the superstar team, their department struggled with ergonomic challenges. Their fixed-height sinks were leading to back pain and poor working postures for a team of people with varying heights. Helton shares: “Our biggest issue was that we have varying heights among our technicians. So, our sink was kind of low. And that was a problem for a lot of our taller technicians, who had to bend over to work at them.” In addition to including height-adjustability into their new sink’s design, Jahnke’s goal was also to update and improve functionality, workflow, and durability compared with the old sinks. According to Jahnke: “We were looking for something that was updated, down to the fact that our sink had been used since 2012. There was some wear and tear on it, old plumbing, and leaks. They weren’t functioning any longer.” The department needed a solution that addressed these concerns and provided an ergonomic space for the team. Consultation Process with Pure Processing Jahnke first learned of Pure Processing through other ambulatory sites within Henry Ford System, which had recently opened and implemented PureSteel™ Healthcare Reprocessing Sinks. The success of these projects, and the satisfaction of the facilities, led to Pure Processing being brought in to help with this project. While the consultation and design process was standardized and streamlined due to previous collaborations, Pure Processing representatives, were “really helpful and easy to talk to”, to according to Jahnke. Having worked on similar projects at other sites, they brought valuable experience and familiarity to the table. However, they faced unique challenges due to the age and size of the building; the decontamination space was limited compared newer buildings on campus. Installation Hurdles Logistics presented significant challenges regarding installation of the sink. The older building wasn’t equipped with a loading dock, which created unique challenges associated with delivering the sink. Additionally, the layout of the building made navigation to the reprocessing space difficult, requiring partial disassembly of some components. Their representative was onsite for the install and helped remedy challenges as they arose. Despite these challenges, Jahnke explained “Anytime we had one of these hurdles and we reached out, my representative was very receptive and made things right. And any issues that had occurred during that time were outweighed by the benefits now from the sink. While the installation was not smooth, it was corrected, and everything worked out.” A New Sink’s Impact on Morale Since implementation of the new PureSteel™ Healthcare Reprocessing Sink in Jahnke’s department, overall satisfaction has been high. “The new sink has been a lot better than our old sink. I hear a lot less complaints about having to be in [decontamination], and it’s a lot more comfortable. It’s so much nicer having the freedom to position our tools and materials where we need them. It’s definitely an improvement for us”, stated Helton. Additionally, the ability to place shelves and necessary items in more convenient locations has had a substantial impact in terms of improved workflow and overall functionality. Helton also stated that there have been some unexpected benefits stemming from the new sink, such as improved lighting, organization, and ergonomics. “Having that overhead lighting definitely made it easier to see into the water to detect leaks. And the pegboard back has been very helpful with all of the different attachments and adjustability. We can move things where we wanted them, we can attach everything to a bin, and put it on the pegboard. So all of that is very helpful, and it’s easy to clean.” Departments come in all shapes and sizes. In the case of Jahnke and Helton’s GI department at Henry Ford Health System Columbus, the age and design of the building created unique constraints that posed challenges to implementation that could have derailed the project. It was the ability to partner with a vendor that could accommodate unique installation challenges and improve not only ergonomics, but functionality, workflow and compliance, that secured Henry Ford a successful new sink installation. Looking to solve some unique problems in your department? We’d love to help!