CATEGORY: Improve Your Department

Jul 10, 2024
Actionable Insights from the 2024 GI Reprocessing Landscape Report
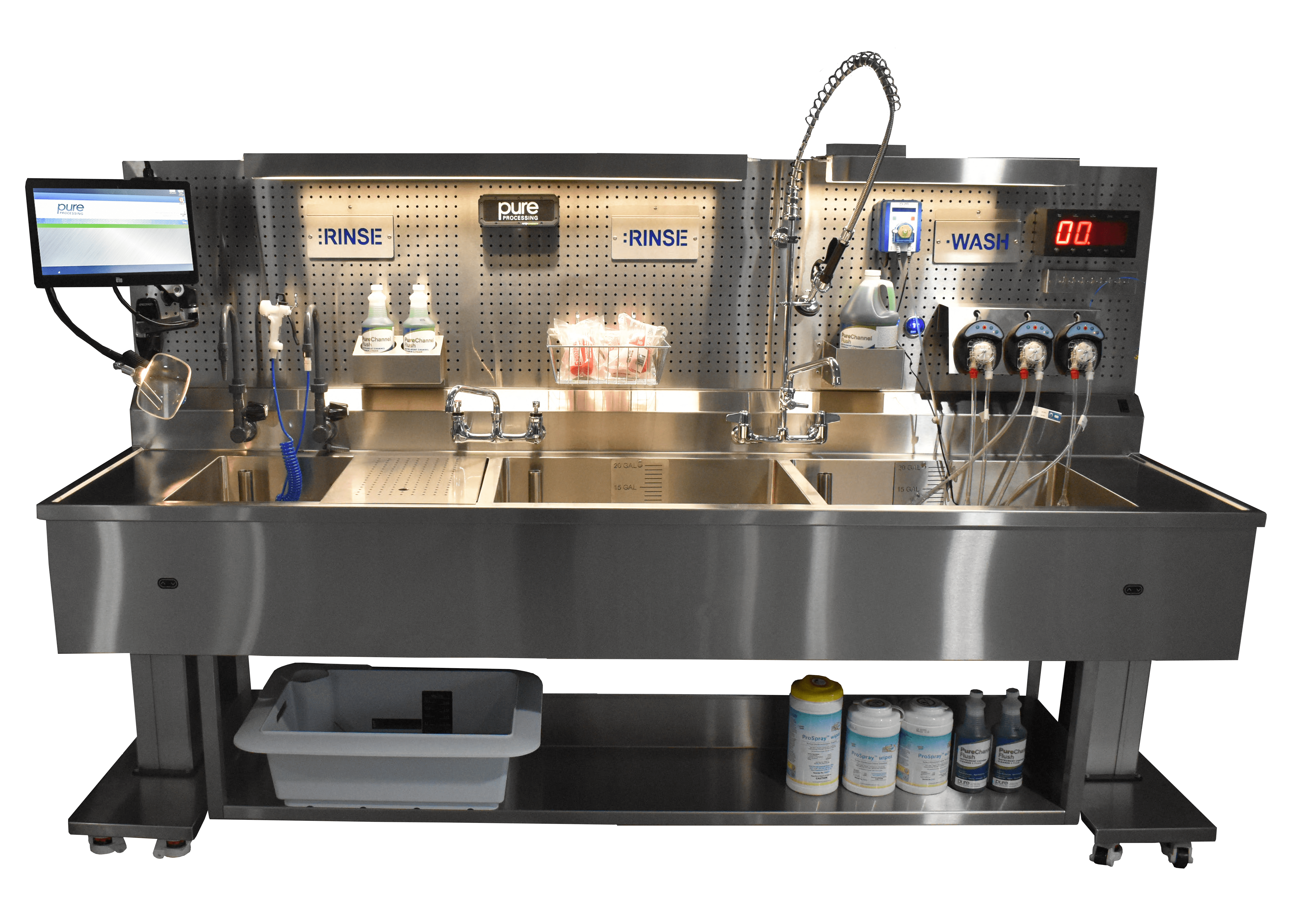
While results from reports like the 2024 GI Reprocessing Landscape Report are interesting on their own, it’s what you’re able to do with the information and data to improve your department that many people are interested in. Here are some actionable insights for RN Nurse Managers, GI department managers and leadership to take from the 2024 GI Reprocessing Landscape Report. Finding new teammates Over 80% of participants in the 2024 GI Reprocessing Landscape Report reported coming from another healthcare-related position prior to entering the GI department. Managers and directors looking to fill vacant roles in their department should continue utilizing their professional network in and outside of their facility, but within the healthcare industry, to find suitable job candidates. 2024 participants cited positions in sterile processing, housekeeping, the operating room, facilities maintenance, and more as previous positions they held before entering GI. Factors that drove individuals to transition to the GI department included: Opportunity to help patients More regular hours Better work/life balance Desire to be more involved in healthcare Interest in the opportunity to learn about and handle GI technology Using these when filling open positions can help leadership fill their candidate funnel and illuminate the importance of the work GI nurses and technicians do. Training & Education Training and education was a popular topic in the 2024 GI Reprocessing Landscape Report, including being among the top three most common, easy to solve, and important to solve problems. It’s clear that GI nurses and technicians are yearning for more opportunities to enhance their industry knowledge and become more effective in their roles. Below are some ideas to address training and education within your department: Speak with your educator about areas of opportunity in your department and what resources can be implemented to help address training needs. Don’t have an educator? Learn how to justify hiring a full-time educator with this blog post. Seek input from your team. Setting aside some time to ask your team what kind of training & education opportunities they would like to see can highlight areas to focus on. Do this during huddles, performance reviews, or during a normal check-in! Talk to your vendors. Vendors (including Pure Processing) offer a wide array of free and easily accessible CE-accredited educational materials. Further, many vendors will happily provide in-services on their tools and equipment, allowing your team to ask questions and get clarity on the more complex aspects of their instrument inventory. Keeping great people 66.50% of participants in the 2024 GI Reprocessing Landscape Report cited their team as being foundational to creating a great endoscopy department to work in. This includes the people they work with, a shared sense of pride and purpose in their work, being patient-focused, and generally fostering a great culture. A few ways to reinforce or develop these characteristics in your department could include: Organizing team building and bonding events to help your team get to know each other outside of their PPE. Finding ways to demonstrate appreciation for what your team does, including the use of programs like Pure Processing’s Citations for Being Awesome. Continuously sharing the importance of your team’s work. One participant shared the following: “I supervise an amazing group of women and work for the best physicians. Most patients are healthy, and procedures are elective. We are helping to prevent colorectal cancers every day!” Listening to their needs. Teams know the pain points and difficulties they face. Using their knowledge and experience to enhance your department demonstrates respect and care for their opinions and needs. Why GI nurses are leaving Expanding on how to keep great teams, it’s important to look not only at what they love about working in GI, but the reasons they opt to leave. 40.11% of participants pointed to working conditions, such as stress, poor ergonomics, and burnout as the top reason for leaving departments. While some of these may be tough to avoid in understaffed departments, improving nurse and technician comfort through ergonomics is more tangible and obtainable. Some considerations include: Implementing tools, such as flushing systems, that reduce or eliminate the need for continued repetitive motion activities, like syringe flushing. Invest in anti-fatigue mats. Long shifts standing on hard tile takes a toll on nurse’s and technician’s knees, hips, and backs. Anti-fatigue mats can contribute to increased comfort over the course of a shift, meaning your team isn’t bringing pain from their shift home with them. Consider the design of the equipment in your department. While capital investment isn’t something that can happen with the snap of your fingers, when the opportunity to upgrade equipment like sinks does arise, consider how design can contribute to the comfort and effectiveness of your team. Learn more about ergonomic design considerations with this blog post. Finding ways to improve your department can feel challenging, especially when less tangible aspects, such fostering a desirable culture or finding the right people when hiring, are what need to be tackled. Using resources like the 2024 GI Reprocessing Landscape Report gives managers a window into what nurses and technicians across the country are looking for and appreciate in their departments, and serve as a great starting point when striving to make changes in your own department. Ready to dive into the report for yourself? Download the 2024 GI Reprocessing Landscape Report for free today!