
Common Problems in Reprocessing: A First-hand Perspective to Address Challenges
-By Hannah Schroeder, BSHA, CRCST, CIS, CHL, CER, Clinical Education Specialist at Pure Processing
We have all been there: one issue unravels a slew of others until you find yourself spiraling and do not know which way is up or where to take the first step towards rectification. The decision paralysis sets in and it’s hard to break out of.
As leaders, it’s always important to ask the following questions:
- What is the main goal we are aiming to accomplish?
- What do we need to address to reach that goal?
Determining answers to these are the secret to identifying your next steps!
It sounds so simple when placed on paper, right? As a former reprocessing educator with a focus in both sterile processing and flexible endoscopy, I know first-hand that a long list of to-dos can be  daunting. The two simple questions asked above hold the potential to become the catalyst for workgroups and committees of stakeholders that can come together to address the problem(s) you’re facing. That problem we all want fixed, we quickly realize is not such an easy fix, after all. Hours of research, planning and implementation are now committed to the process improvement plan set forth.
daunting. The two simple questions asked above hold the potential to become the catalyst for workgroups and committees of stakeholders that can come together to address the problem(s) you’re facing. That problem we all want fixed, we quickly realize is not such an easy fix, after all. Hours of research, planning and implementation are now committed to the process improvement plan set forth.
But as someone who has experienced the daunting task of addressing issues in reprocessing departments, I also understand the support that comes with workgroups and the importance of focused and careful planning that allows for successful prioritization and execution of process improvements.
In our 2023 GI State of the Industry Report, the following issues were identified as the Top 3 most common problems with scope reprocessing:
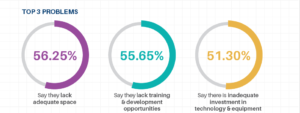
Furthermore, participants also offered insights into what they considered to be the easiest problem to solve. The top 3 were:
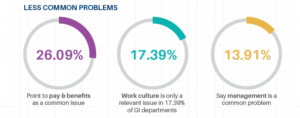
Many of the participants indicated that education and technology updates were both common and easy to fix, while compliance was added to the ranks as an easy to fix problem. Take heart! With a closer look you will often find that these “problems” are intricately connected and addressing one could improve another. For example, compliance and education can go hand in hand, while improved technology can also lead to improved compliance.
Though you may not have the chance to expand your reprocessing department now, and at one point, neither did I; I can share some ways to address these common problems with the resources available today.
Training & Compliance
We cannot comply with what we’re unaware of. That rings true for managers and technicians alike. Some practical ways I found to supply teams with information and education included sharing resources such as websites, blog posts and regulatory announcements, as well as, ensuring routine in-services were scheduled. This included refresher courses on equipment they used, as well as topics like quality and ergonomics with our safety departments.
In tandem with compiling and sharing educational resources, it is important to make education an expectation as well. Carving out time even just a couple of days a week, whether it be ten minutes at the start or end of their shift, or designated time during a staff meeting, education and training can be woven into even the most demanding of days.
The more interactive the education was, the better engagement I had with my team(s). An example of this included breaking down the ANSI/AAMI standards and presenting the content during staff meetings. With a single recommendation on the screen, I prompted the team to discuss how our department was currently compliant and explored the resources available enabling them to achieve that compliance. This also created opportunities for the team to discuss process improvement ideas.
Technology, Training & Compliance
Technology improvements can range from automated flushing devices to tracking system updates. Technology can automate processes, reduce human error, and improve department outcomes. 
Many tracking systems now provide options for including documents, alerts, and notes to remind the user about specific reprocessing steps, or new processes that have been added or updated.
Providing the ‘why’ behind updates and innovative technology, and how it enhances our ability to achieve compliance, provides the team with the contextual information that creates buy-in and engagement across the department.
To do this effectively, it was critical to stay up on guidelines and standards, as well as explore emerging products and technologies. Fostering relationships with ancillary departments and networking with other hospitals and manufacturing companies kept the conversation going and provided helpful insight that may not have otherwise been considered. Taking time to meet, observe and explore different options kept the creative wheels turning and kept our department aware of different capabilities and how we could effectively apply them.
Technology, Equipment & Space
When space is tight, the equipment and technology we bring into our reprocessing spaces matter. Additionally, the way equipment and tools were staged and configured had a significant impact on the space as well. Whether it’s high-density shelving or leaning out supplies that no longer serve a purpose, there are countless ways to optimize the space a department has to work with.
One other exercise that I used was streamlining processes and workflows to reduce overall foot traffic and ensure team members were not crossing paths and bumping into each other. Adjusting workflows does not always give back space, but it can change how the end user navigates and functions within the space provided.
Conclusion
Immerse yourself in the work and get a firsthand look at the processes and pain points being experienced daily helps you as a leader relate to your team and get the wheels of creativity spinning. Providing your own context to the data and ratings can guide your process improvement initiatives and you too will find that addressing one issue may just improve the outcomes of others. It is easy to become overwhelmed by the scope of a project, but understanding the influence each process has on the overall picture allows you to see that even the smallest change can lead to big outcomes!
Interested in learning more? Check out these space-related blog posts:
Workstation Disorganization: Problems and Opportunities
Author Profile
- Courses with CE credits
- Local chapter meeting presentations & workshops
- Assistance with Pure Processing product purchase and deployment, for both your operational and capital purchases




