Endoscope Manual Cleaning: A Fragile Chain
“A chain is only as strong as its weakest link.”
In an environment where manual cleaning is foundational, this quote is a fitting description of our endoscope reprocessing practices. Routinely auditing our processes keeps us apprised of the ‘weakest link’, where inconsistencies, interpretations and best guesses can negatively impact the quality of care.
Manual cleaning relies heavily on the focus, tenacity and consistency of our sterile processing and endoscopy technicians, all of which can be influenced by disruptions or distraction, often times, outside of their control. When it comes to our manual processes, it’s important to create robust practices that both provide consistency and support our reprocessing professionals, across every endoscope, every time.
In the 2025 GI Landscape Report, 39.37% of participants indicated that IFU compliance was a top consideration for their department endoscope practices. So when it comes to our manual cleaning processes, where do we audit to identify gaps?
Cleaning Chemistries
Cleaning chemistries, including enzymatics and detergents, have dosing recommendations that need to be adhered to for appropriate and safe concentration, as well as effective cleaning.
Point of Weakness: In a place where more isn’t always better, too much enzymatic can leave behind residue. In the same vein, underdosing can reduce cleaning effectiveness leaving behind bioburden and gross soil. Manually dosing provides room for under of overdosing.
Soak Times
Just as much as dosing the right amount of enzymatic or detergent is crucial, so is the appropriate contact time. Contact time ensures

chemistries have a chance to break down heavily soiled or dried bioburden properly.
Point of Weakness: Soaking times can be determined by both/either the chemistry manufacturer IFU or the medical device IFU. The practice of contact and adequate soaking times rely heavily on kept time and alerts to verify and affirm the right time requirements have been met to achieve the intended results. Facilities without timers or alerts run the risk of breaching soaking IFU.
Brushing
Flexible endoscope channels are long, dark and may be damaged. It’s a place that cannot be easily viewed during the manual cleaning process. Adequate brushing techniques are a must and understanding how brushes work better equips technicians to use them appropriately.
Point of Weakness: Brush contact with the internal channels is done without immediate visual verification, and though flexible endoscope IFU can provide guidance to include brushing until the brush comes clean, the results are still arbitrary and assumed.
Flushing
Flushing channels is a crucial step in the manual cleaning process. Copious amounts of cleaning solutions, water and critical rinse are

essential for effective reprocessing.
Point of Weakness: Manual flushing presents ergonomic strain from the repetitive motions of push and pull to fill and express the syringe when cleaning channels. The strain effects consistency and technician endurance as muscles fatigue during repetitive movements. Consequently, this also leads to variance in pressure and volume being released from the syringe during the flushing step.
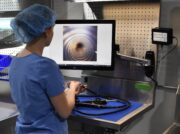
Inspection
Cleaning verification and inspection continues to gain traction in flexible endoscope reprocessing. Flexible endoscopes have many “hidden” areas where bioburden can be difficult to remove, and lead to adverse effects to both the endoscope and the patient.
Inspection incorporates various types of tools and resources including lighting, magnification, borescopes and even cleaning verification tools that measure organic residues such as protein or ATP. Each tool has its own unique intent and purpose.
Point of Weakness: Without adequate and fully comprehensive inspection tools, verification of effective cleaning is near impossible. Magnification can be an aid, but doesn’t visualize internal components. Additional lighting can improve visualization but doesn’t offer focused precision viewing. Endoscope workflows that lack comprehensive quality assurance (QA) steps risk bioburden reside inside channels.
Guess work will break down processes and quality in a swift motion, with an impact that reaches far beyond the decontamination sink. It breaks down each process that follows and if not caught or identified before storage, can lead to adverse effects in the procedure. Knowing where our weakest links are and improving them sets us up for a stronger processes, sustainable practices and quantifiable assurance of quality outputs.
Curious to learn more about trends and additional insights into flexible endoscopes reprocessing? Download our 2025 GI Landscape Report, here. You can earn 1 free CE credit, too!