
Integrating ergonomic tools to reduce injury and strain in packaging and assembly
Improve productivity, reduce staff injuries, and enhance patient safety with a few simple tools
Medical devices or instruments go through numerous reprocessing steps before they’re deemed sterilized. The packaging and assembly area of the sterile processing department (SPD) is critical in assuring “the sterility of an item” while protecting “the contents until use.” To assure sterility, personnel must follow standards and guidelines to make sure the packaged instrument will not pose risks to patients.
The rigorous requirements for preparation for sterilization in ANSI/AAMI ST79:2017 requires that “devices should be cleaned; dried; inspected for cleanliness, flaws, and damage; assembled; and packaged according to the manufacturer’s written IFU.”1 To do so, ANSI/AAMI ST79 states that “there should be sufficient space for clean textile storage (both before and after assembly into packs), an illuminated inspection table, and patching equipment. …the clean work area should include space for magnifying lights; processing tables, which should be made of nonporous materials (e.g., stainless steel), ergonomic, and, preferably, height-adjustable.”
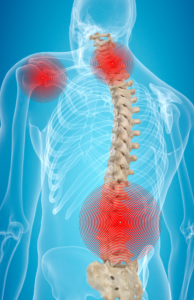
The effects of packaging and assembly on the musculoskeletal system
The packaging and assembly process during medical device reprocessing is often very labor-intensive and can lead to musculoskeletal disorders (MSDs). Many times, departments have the best intentions when designing and establishing a packaging and assembly area, only to realize a few more options would have helped improve ergonomics and overall employee satisfaction.
 Meet ergonomic compliance
Meet ergonomic compliance
ANSI/AAMI ST79 and OSHA ergonomic guidelines2 require height-adjustable workstations. Sterile processing trays are often extremely heavy. Bending and lifting trays onto workstations that are not at comfortable working levels can lead to musculoskeletal disorders. According to OSHA, MSDs “affect the muscles, nerves, blood vessels, ligaments and tendons. Workers in many different industries and occupations can be exposed to risk factors at work, such as lifting heavy items, bending, reaching overhead, pushing and pulling heavy loads, working in awkward body postures and performing the same or similar tasks repetitively. Exposure to these known risk factors for MSDs increases a worker’s risk of injury.”
The repetitive motion tasks that are also associated with packaging and assembly can lead to injury and strain. Did you know that ergonomic options go beyond making workstations height-adjustable?
Reduce injuries from repetitive tasks
 Keeping items easily within reach is key to reducing musculoskeletal disorders from repeated reaching. By employing an integrated pegboard wall, shelving, bins, peel pouch rolls and trays, tape dispensers and more can be designed with the user in mind. Pegboards allow items to be easily moved and adjusted to meet the end users physiological and workflow needs.
Keeping items easily within reach is key to reducing musculoskeletal disorders from repeated reaching. By employing an integrated pegboard wall, shelving, bins, peel pouch rolls and trays, tape dispensers and more can be designed with the user in mind. Pegboards allow items to be easily moved and adjusted to meet the end users physiological and workflow needs.
When it comes to workflow, “providing adequate space for supplies and equipment and designing the layout to facilitate the flow of work through the various steps of preparation contributes to the efficiency and accuracy of the sterile processing staff.”3
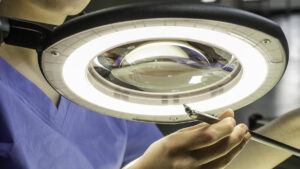
Meet lighting compliance
 In 2013, researchers found that by integrating a few simple ergonomic changes into a department’s design can help improve staff safety. In one department, “a lack of space in the packing area,” led to “…undesirable twists when moving around equipment.”4 By leaving “enough space to establish safe and sound work processes,” the department’s workflow improved.
In 2013, researchers found that by integrating a few simple ergonomic changes into a department’s design can help improve staff safety. In one department, “a lack of space in the packing area,” led to “…undesirable twists when moving around equipment.”4 By leaving “enough space to establish safe and sound work processes,” the department’s workflow improved.
Another area in which prep and pack table design can benefit the end user is integrated lighting. ANSI/AAMI ST79: 2017, 3.3.5.6 states that “adequate lighting of work surfaces should be provided in accordance with the recommendations of the Illuminating Engineering Society of America (IES) for minimum levels of illumination…” The rationale is based on the “importance of speed or accuracy of the work done in the area (the greater the importance of speed or accuracy, the more illuminance needed).”
Upon completion of the cleaning process, staff must perform cleaning verification. “Cleaning verification by users should include (a) visual inspection combined with other verification methods that allow the assessment of both external surfaces and the inner housing and channels of medical devices…”
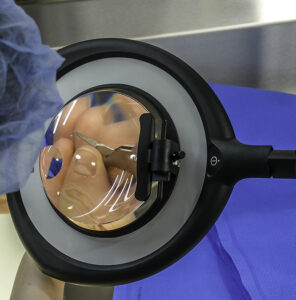 Many instruments have very small pieces and tips that look quite similar to the naked eye. It is even more difficult to notice these subtle differences under inadequate lighting. A 2019 study regarding errors in packaging surgical instruments found that “personnel error is the primary reason for packaging errors. Central sterile supply department (CSSD) staff members are not familiar with the clinical utilization of surgical instruments, and therefore it was hard for them to distinguish between instruments with minor differences.”5
Many instruments have very small pieces and tips that look quite similar to the naked eye. It is even more difficult to notice these subtle differences under inadequate lighting. A 2019 study regarding errors in packaging surgical instruments found that “personnel error is the primary reason for packaging errors. Central sterile supply department (CSSD) staff members are not familiar with the clinical utilization of surgical instruments, and therefore it was hard for them to distinguish between instruments with minor differences.”5
To avoid errors in packaging surgical instruments, departments can meet compliance with magnifying task lighting. Magnifying task lights improve patient safety by enhancing visual inspection with direct light, as well as allowing technicians to verify cleaning processes and assess any damage to instruments prior to sterilization. Of equal importance, magnification and lighting improve staff safety by reducing eye and neck strain.
Find sterile wrap perforations without eye strain
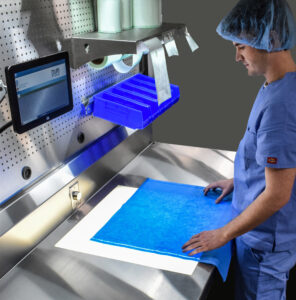 Additionally, when wrapping trays with woven and nonwoven sterile wraps, ANSI/AAMI ST79:2017, 9.5 states that departments must “inspect the wrap to ensure that it is free of defects that could have an adverse effect on the performance of the material.”
Additionally, when wrapping trays with woven and nonwoven sterile wraps, ANSI/AAMI ST79:2017, 9.5 states that departments must “inspect the wrap to ensure that it is free of defects that could have an adverse effect on the performance of the material.”
The International Association of Healthcare Central Service Materiel Management (IAHCSMM) recommends that visual “inspection is performed using a light table that has a light source built into the tabletop to help spot small holes and punctures. As the wrap is passed over the lighted table top, light shines through the small holes and punctures making them easier to identify.”6 By doing so, imperfections and perforations in sterile wrap materials can easily be identified and staff do not have to strain to find small holes.
Reduce medical costs while increasing productivity and patient and staff safety
In 2008, researchers sought to connect the costs of injuries and strains on a sterile processing department and in turn, design an intervention to alleviate the number of injuries and straining. The study found that in this particular department, “between 2001 and mid-year 2005, employees in the sterile processing center (SPC) experienced 32 injuries, costing $187,266.00 in direct medical costs (i.e., loss expenses), with strain injuries accounting for 94% of the total expenses and 50% of the total injuries.”7 Many departments are already backlogged with cases and need capacity boosting solutions. Adding injuries and strain to staff only creates further backlogs. By integrating ergonomic solutions in the packaging and assembly area, costs are reduced and throughput is increased. Not to mention, staff productivity and employee satisfaction are boosted as well.
Learn more about Pure Processing height-adjustable workstations and inspection tables to meet your workflow and workplace environment goals.
Want to earn 1 free CE? Watch Under Wraps: Packaging Materials, Their History, Efficacy and Creating Packaging Material Inspection Protocols
References
- Association For The Advancement Of Medical Instrumentation. Sterilization Standards Committee, Association For The Advancement Of Medical Instrumentation, & American National Standards Institute. (2017). Comprehensive guide to steam sterilization and sterility assurance in health care facilities. Arlington, Va: Association For The Advancement Of Medical Instrumentation.
- Ergonomics – Overview | Occupational Safety and Health Administration. (n.d.). Retrieved February 3, 2021, from www.osha.gov website: https://www.osha.gov/ergonomics
- Association For The Advancement Of Medical Instrumentation. Sterilization Standards Committee, Association For The Advancement Of Medical Instrumentation, & American National Standards Institute. (2017). Comprehensive guide to steam sterilization and sterility assurance in health care facilities. Arlington, Va: Association For The Advancement Of Medical Instrumentation.
- Hall-Andersen, L. B., & Broberg, O. (2014). Integrating ergonomics into engineering design: The role of objects. Applied Ergonomics, 45(3), 647–654. https://doi.org/10.1016/j.apergo.2013.09.002
- Zhu, X., Yuan, L., Li, T., & Cheng, P. (2019). Errors in packaging surgical instruments based on a surgical instrument tracking system: an observational study. BMC Health Services Research, 19(1). https://doi.org/10.1186/s12913-019-4007-3
- International Association Of Healthcare Central Service Material Management. (2016). Central Service Technical Manual. (p. ). : IAHCSMM.
- Boynton, T., & Darragh, A. R. (2008). Participatory ergonomics intervention in a sterile processing center: a case study. Work (Reading, Mass.), 31(1), 95–99. Retrieved from https://pubmed.ncbi.nlm.nih.gov/18820424/







