What to know about: Ocular Reprocessing
Nearly everyone agrees that proper cleaning and sterilization of any kind of procedural instrument is vital within a surgical setting to ensure the best patient outcomes. However, it’s easier said than done when the instruments are more complex, and guidelines also become tedious and require instrument-specific reprocessing steps.
Ocular devices are an example of devices fitting the complexity criteria. Departments must be prepared to have a detailed discussion on how to reprocess these devices correctly to reduce potential infections and aid in a safe surgical experience.
There are many reasons why ophthalmic devices are tricky to clean. In this post we are going to cover three (3) questions to consider when assessing ocular reprocessing practices.
- What are the potential consequences of improper ocular instrument cleaning?
- How should care and cleaning instructions be modified?
- How do IFU inform reprocessing decisions?
What are the potential consequences of improper reprocessing?
Mainly associated with cataract surgeries, any ocular surgery where the anterior segment is exposed presents the risk of a Toxic Anterior Segment Syndrome (TASS) infection, an acute inflammatory response to foreign material inside the anterior chamber of the eye that can lead to severe vision impairment.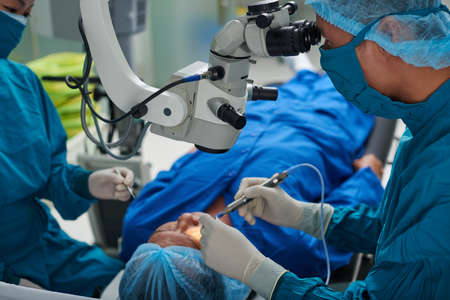
Incomplete cleaning processes that leave residual cleaning chemistries, mineral deposits from steam sterilizers, and/or powder from sterile gloves have contributed to cases of TASS outbreaks.
TASS has often been linked to the failure to follow the processing procedures recommended by the manufacturer or by guidelines such as ANSI/AAMI ST79, AORN 2010a, CDC 2003b, HSPA.
Other notable transmitted diseases have included HIV, hepatitis C Virus and prions such as Creutzfeldt-Jakob disease (CJD). (TJC, 2019)
For these reasons, care and cleaning instructions are often modified for ocular devices.
How is care & cleaning impacted?
Pre-cleaning in the OR
Flushing ophthalmic lumens should be initiated in the OR and completed in the decontamination area. When sterile water baths are used for cleaning or soaking soiled instruments in the OR, they should be separated from the sterile field and instruments still in use. When flushing is used as part of a cleaning technique, the effluent should be discharged into a sink or separate basin while minimizing splash and aerosolization, so that contaminated fluid is not spread.
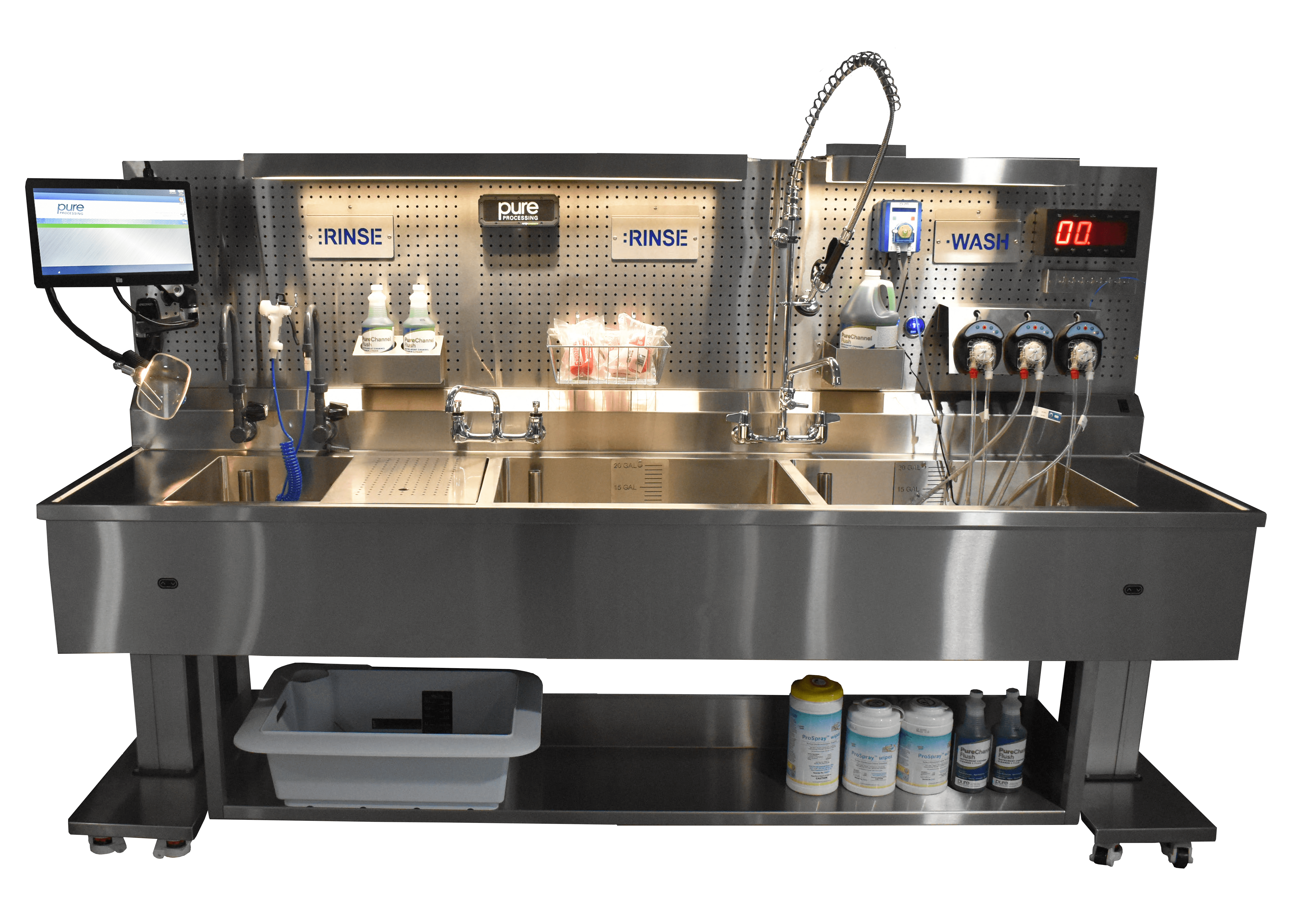
Dedicated processing areas
Many guidelines recommend that ocular instruments are best processed in dedicated areas of the department solely for eye instrumentation. Ophthalmic device IFUs may, for example, restrict the use of enzymatic detergents. When residual enzymatic gets in a patient’s eye during a procedure, it can lead to blindness or other severe, negative outcomes. You may also find your ocular IFU now includes single-use brushes only, or specific water quality requirements.
Water quality
The volume and type of water for cleaning and rinsing should follow the manufacturer’s IFU. The IFU for many intraocular instruments recommend or require critical water (sterile distilled, reverse osmosis, or deionized) for most cleaning steps and for final rinsing. Use of tap water for rinsing and for removal of detergent should be used only if in compliance with the manufacturer’s IFU for the detergent and for the equipment. Because tap water can contain heat-stable endotoxins from gram-negative bacteria found in a municipal water supply, critical water is recommended for the final instrument rinse.
Enzymatic detergents
Cleaning with enzymatic detergent may be warranted in certain situations. If enzyme detergents are used for any reason, instructions for proper dilution and disposal of cleaning solutions should be followed. The cleaning solution should be mixed with measured amounts of water according to the detergent’s IFU. The instruments should be thoroughly rinsed to ensure removal of all cleaning agents as well as all debris loosened during the cleaning process.
Review your ocular instruments manufacture instruction for use (IFU) for information on recommended cleaning chemistries and compatibility.
Ultrasonic systems
Ultrasonic cleaning poses another risk of TASS infection, according to the TASS Task Force surveys. If an ultrasonic cleaner is used, the technician should remove all visible soil before placing instruments in the ultrasonic cleaner.
How do IFU’s inform reprocessing decisions?
In years past as a result of IFU revisions, ocular devices were required to have their own dedicated ultrasonic cleaners. Many are now discussing the potential for two dedicated ultrasonic systems. One dedicated for static soaking and ultrasonic cleaning, while the second for providing  critical water for ultrasonic rinsing.
critical water for ultrasonic rinsing.
Ocular reprocessing IFU may also soon include a restriction to singe-use brushes for cleaning. Inventory that is a mix of single-use and multiple-use brushes would need to be separated, so single-use items are not accidently reprocessed or re-used.
When it comes to automated cleaning, IFUs can include specifics regarding washer cycles and parameters. Ocular instruments are delicate and may require a gentler cycle parameters.
Reaching out to your local washer/disinfector rep will help you determine cycle capabilities and what adjustments can be made to comply with ocular reprocessing IFUs.
These potential changes to IFU demonstrate why regular review of guidelines and IFU is crucial to maintaining compliance. Changes can be implemented and easily slip by. It may also be recommended to partner with other departments who reprocess similar specialties, and rely on each other for updates, news, and changes to guidelines and IFU.
Understanding potential consequences, completing step-by-step cleaning according to guidelines, and making a continuous effort to review IFU and guidelines will lead to a cleaner and safer surgical experience for both patients and reprocessing staff. Ocular reprocessing may pose a challenge, but it certainly can be managed.
Pure Processing provides a portfolio of solutions for departments responsible for ophthalmic reprocessing. Contact us to see how we can help create automated flushing systems, dedicated reprocessing areas, and enhance your compliance and efficiency.
Sources Cited
https://www.aao.org/clinical-statement/guidelines-cleaning-sterilization-intraocular
https://cdn.hpnonline.com/files/base/ebm/hpn/document/2021/02/2103_CEU.602bd67277ea1.pdf