3 areas to improve during the manual cleaning phase of medical device reprocessing
Flushing, visual inspection/cleaning verification, and soaking are three of the most vital processes in medical device reprocessing in reducing healthcare acquired infections (HAIs) and ensuring patient safety. When these processes are not performed against standards and guidelines, bioburden can easily be left on an instrument for the next patient.
3 areas that need improvement
Soaking
Items should be pretreated with an initial cold water rinse with running tap water or an initial soak in cool water and/or a clinical-soil-dissolving pretreatment product (e.g., an enzymatic cleaner or pH neutral detergent). –ANSI/AAMI ST79:2017, 7.6.1
It is well known that the speed in which processes are performed and completed, impact the patient care environment. When robotic instruments and scopes require long soaking times per manufacturer’s instructions for use (IFU) or from delayed reprocessing, maintaining surgical schedules can become jeopardized.
As a result, larger instruments such as robotic devices or flexible scopes, that have IFU soaking requirements, utilize lots of basin space, reducing the amount of space sterile processing departments have to perform other, necessary pre-cleaning functions. The ability for technicians to multitask while instruments are soaking becomes impossible when basin space is not available. And ultimately, without proper soaking, gross soils are not properly broken down, making it difficult to remove bioburden during the cleaning process.
Flushing
Thoroughly flushing lumens helps ensure complete surface contact with the solution. If a brush is too large, it will not fit into the lumen; if it is too small, it will not have complete contact with the lumen walls and, consequently, will not clean them thoroughly. –ANSI/AAMI ST79:2017, 7.6.2
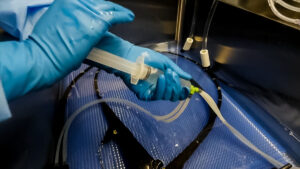 While syringes provide a simple, as well as disposable option when flushing, they aren’t entirely effective at removing bioburden. Using a syringe to pull liquid and plunge it into instruments multiple times is not productive. Furthermore, no two techs flush exactly the same way with syringes or spray guns, leading to inconsistent flushing practices. As a result, “copious flushing” is variable.
While syringes provide a simple, as well as disposable option when flushing, they aren’t entirely effective at removing bioburden. Using a syringe to pull liquid and plunge it into instruments multiple times is not productive. Furthermore, no two techs flush exactly the same way with syringes or spray guns, leading to inconsistent flushing practices. As a result, “copious flushing” is variable.
Flushing instruments with spray guns is another inefficient cleaning method. Technicians are not able to clean more than one instrument channel at a time, which affects a central sterile processing department’s throughput. Compromised productivity isn’t the only disadvantage when it comes to flushing with spray guns. Spray guns create aerosols, making contaminated liquids even more dangerous when they are converted to vapors, coating staff members in fluids and hard-to-reach areas, putting staff and patients at risk.
Visual inspection/cleaning verification
Instruments should be carefully inspected for flaws, damage, debris, detergent residue, and completeness… –ANSI/AAMI ST79:2017, 7.4.1
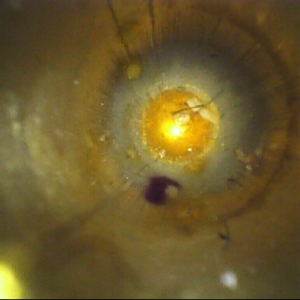
Endoscope interior
The importance of visual inspection cannot be overstated. The naked eye is unable to detect microscopic bioburden, minute cracks or damage in instruments, even lint inside of lumened channels. If bioburden is left in the channel, the chance of the next patient developing a HAI increases. In 2015, 687,000 patients developed HAIs in the U.S. Of that number, 72,000 of those patients died.1
Visual inspection can be a costly investment. The alternative leaves sterile processing and endoscopy departments to visually inspection instruments and scopes with the naked eye and/or magnification lenses. Unfortunately, eyes and magnification lenses can only visualize easy-to-reach areas in channels. In those hard-to-reach areas of channels, a lot of patient safety risks lurk.
3 Ways to Improve Pre-Cleaning Processes
 1. Create dedicated soaking spaces
1. Create dedicated soaking spaces
Renovating a decontamination area is costly and not always necessary. Soaking robotic arms, scopes and other larger instruments that demand precious sink basin space also hamper efficiency and the bottom line. Utilizing tools such as smaller mobile sinks, and soaking containers, departments can improve turnover times while meeting compliance.
For example, smaller mobile options, such as the PureSteel Mobile Soaking Station, feature a soaking basin that is wide enough to accommodate soaking requirements for robotics and larger instruments. Its easy-to-drain options also do not require plumbing and can be outfitted with numerous accessories.
Mobile carts that feature vertical storage for multiple soaking containers are built specifically with soaking in mind. By utilizing vertical soaking space, numerous devices can be soaked in containers that are designed to fit the device, as well as easily transport and drain the containers in the smallest overall footprint available.
 2. Automate flushing
2. Automate flushing
By utilizing an automated flushing pump, precleaning compliance is achieved through friction, fluidics and contact time with detergents. Automated flushing systems such as the FlexiPump Independent Flushing System, achieve these three objectives through pressurized, copious flushing for specified periods of time. The adaptability of automated flushing systems to connect to virtually any lumened device, allows for a tight connection and a complete flush each time.
Automated flushing also streamlines processes by allowing technicians to multitask. Automated flushing systems can connect and flush multiple devices or ports at once, freeing up technicians to brush complex pieces on devices, clean the surrounding work areas, or start prepping the next tray for reprocessing.
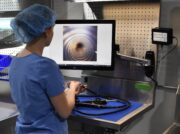
3. Implement visual inspection technology
The best visual inspection intentions are not going to ensure 100% verification of cleaning practices without the flexibility that inspection borescopes offer. Visual inspection borescopes come in many different lengths and diameters that can provide visualization in areas that are hard-to-reach. Inspection scopes are available in fiber-optic and digital models for various resolution needs.to inspect channels, connection ports, distal tips and spaces behind elevator channels. Inspection borescopes can be easily manipulated to identify debris, bio-burden, damage and irregularities that the naked eye and magnification lighting cannot achieve.
borescopes offer. Visual inspection borescopes come in many different lengths and diameters that can provide visualization in areas that are hard-to-reach. Inspection scopes are available in fiber-optic and digital models for various resolution needs.to inspect channels, connection ports, distal tips and spaces behind elevator channels. Inspection borescopes can be easily manipulated to identify debris, bio-burden, damage and irregularities that the naked eye and magnification lighting cannot achieve.
To aid technicians in visual inspection, borescopes can also be attached to a monitor so that the borescopes camera can be displayed in larger resolutions to get an even more detailed analysis of cleaning verification, exceeding visual inspection compliance.
By implementing these processes, departments can improve compliance, reduce the risk of HAIs, and become more efficient. Analysis of current departmental practices, manufacturer IFUs, and standards and guidelines, can help create a best practices protocol improve staff and patient safety.
To learn how to create protocols and to earn 0.5 CEU, watch A Royal Flush: Your Winning Hand for Pre-Cleaning Protocol.
References
- https://www.cdc.gov/hai/data/portal/index.html#:~:text=From%20the%20HAI%20Hospital%20Prevalence,survey%20to%20have%20an%20HAI.