Solutions for Easily Boosting Capacity in Backlogged Sterile Processing Departments
 It is a scene that is all too familiar in sterile processing departments: case carts pilling up in decontamination, while scheduled surgeries are delayed or postponed due to backlogs in reprocessing. Those backlogs are often created by bottlenecks that can easily be adjusted or eliminated with a few uncomplicated solutions.
It is a scene that is all too familiar in sterile processing departments: case carts pilling up in decontamination, while scheduled surgeries are delayed or postponed due to backlogs in reprocessing. Those backlogs are often created by bottlenecks that can easily be adjusted or eliminated with a few uncomplicated solutions.
Sterile processing backlog area 1: Soaking
The most common sterile processing bottlenecks occur during the soaking and manual cleaning processes.
Soaking and manual cleaning is critical for patient safety. ANSI/AAMI ST79: 2017, 7.5.1 states “presoaking instruments moistens and loosens the soil, thus making the cleaning step more effective and efficient.”1 Additionally, device instructions for use (IFU), such as robotics, can require soak times of up to 30 minutes. Flexible and rigid scopes can be even more demanding when delayed reprocessing occurs, requiring a minimum of one hour of soaking, and up to ten hours for some gastrointestinal scopes.²
Many reprocessing departments do not have enough sink basins to dedicate tor instruments with extended soak times. Additionally, many sink basins are not wide enough to accommodate soaking larger instruments or trays, leading to more reprocessing delays.
Robotic instruments and scopes are inherently difficult to clean. Properly soaking the instrument loosens and removes gross bioburden to make the cleaning and rinsing process more effective. If proper protocols are not followed during the manual cleaning process, that can add additional delays to an already bottlenecked process. So how do departments prioritize basin space and while meeting standards and guidelines?
Options to accommodate medical device soaking requirements

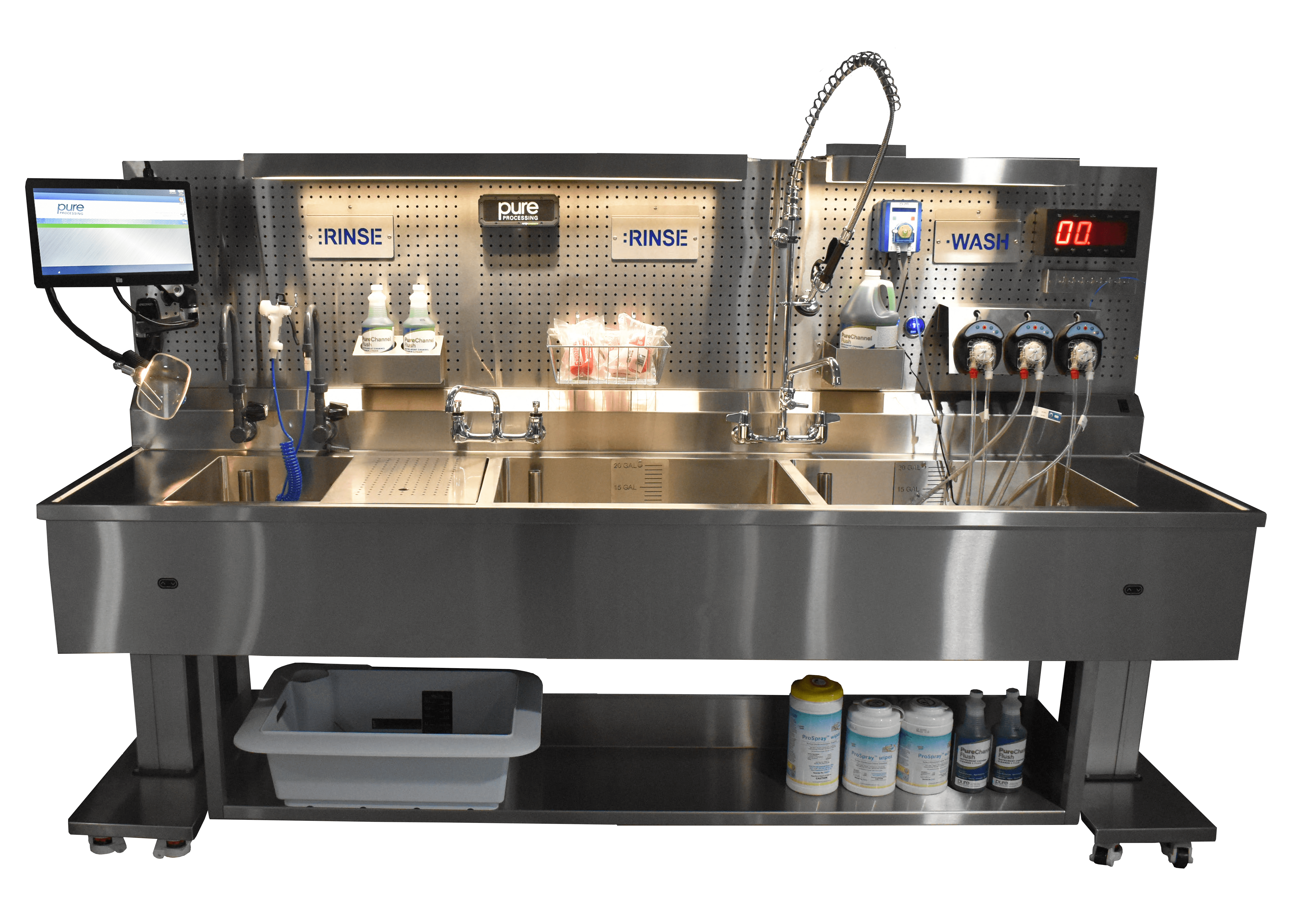
Mobile carts are a quick fix for departments that do not have extra space or resources for a renovation. Departments can create a dedicated soaking space during peak processing times by incorporating a sink insert into a mobile cart or integrating a mobile sink basin. Sink inserts and mobile carts are available in a variety of sizes to accommodate robotics, rigid and flexible scopes, as well as separate areas for ocular instrument reprocessing.
Mobile carts are available in multi-tier options so that departments can soak multiple sets of instruments at once within a small footprint. The mobility of the carts also makes it easy to store the cart and sink inserts when not in use or utilize it for vertical storage options as a place to store soaking containers when not in use.
Sterile processing backlog area 2: Flushing
Properly flushing instruments during the manual cleaning process can create backlogs when volume of lumened instruments and processes for flushing are considered.
Lumens must be flushed with highly efficient practices, so time-saving measures can be difficult to implement without the expense of quality. Contact, friction, and fluidics are necessary to properly remove bioburden from lumened instruments and scopes.
- Specific contact times are required by detergent IFUs to effectively dissolve and loosen bioburden deposits such as blood, fat, and tissue
- Friction, by brushing or flushing, facilitates the separation of bioburden from its points of attachment on internal device surfaces
- Often, brushing or flushing alone does not remove hard, stuck-on bioburden. By integrating fluidics, or a high volume of fluid under pressure, those volumes ensure that detergents and residues are completely flushed from interior channels and lumens
Traditional tools to flush lumened devices such as syringes and spray guns may be a simple, cost-effective option. However, cost-effectiveness should be balanced with efficiency and effectiveness.
Syringes may be a simple, traditional solution. But pulling liquid and plunging it into each instrument for a specified number of times is a time-consuming process. And no two technicians’ flush instruments in the same way, every time. That can lead to practice discrepancies from shift to shift.
Similar to syringes, spray guns cannot clean more than one channel at a time, and require technicians to use both hands, which decreases the ability for technicians to multi-task.
Alternatives to flushing with syringes and spray guns
 Automated flushing systems effectively address the issue of throughput by enabling simultaneous flushing for multiple channeled items or ports with much less connection or disconnection time. One automated system can allow technicians to flush up to 5 items at a time. Output can be doubled or even tripled when multiple systems are installed at sinks.
Automated flushing systems effectively address the issue of throughput by enabling simultaneous flushing for multiple channeled items or ports with much less connection or disconnection time. One automated system can allow technicians to flush up to 5 items at a time. Output can be doubled or even tripled when multiple systems are installed at sinks.
By automating the flushing process, departments can also ensure that devices are consistently flushing at the proper volumes per instrument IFU and ANSI/AAMI ST79:2017 flushing and rinsing standards. Automated systems provide options for flushing for specific amounts of time at specific volumes so that contact time IFUs are met. Additionally, treated and untreated water and cleaning solutions can be integrated into the system to aid in loosening bioburden and assuring pre-cleaning protocols are met.
Automated systems are adaptable to a wide variety of instruments:
- Orthopedic
- Endoscopic
- Robotic
- Ocular
- Laparoscopic devices
- Suction tips
- And more
These flushing systems ensure automated, consistent flushing for your full channeled instrument inventory.
Reducing reprocessing bottlenecks NOW
When departments are already challenged with time constraints, fast, affordable solutions are the easiest way to increase capacity. Large-scale renovations are not always necessary. Instead, simple tools such as mobile carts, sink inserts, and automated flushing systems can be easily installed and implemented to alleviate backlogs and reduce processing times. More care can be taken to deliver safer outcomes for staff, instruments, and patients when departments do not feel rushed to provide instrumentation quickly.
Explore mobile cart, sink insert, and automated flushing system options to increase your sterile processing capacity!
Follow us on Facebook and LinkedIn for more educational content!
Looking for more blog topics like this? Read 3 Ways Height Adjustable Sinks and Tables Improve Sterile Processing Workflow
References:
- ANSI/AAMI ST79:2017 – Comprehensive guide to steam sterilization and sterility assurance in health care facilities. webstore.ansi.org. Accessed November 17, 2020. https://webstore.ansi.org/Standards/AAMI/ANSIAAMIST792017?gclid=Cj0KCQiAhs79BRD0ARIsAC6XpaWiXjfqTZozgNVWCjY53KHU_bD9gvHr7wsoeypRrbhjCxnrk7kqeR0aAnVkEALw_wcB
-
Benedict M. Delays in Endoscope Reprocessing … and the Biofilms Within. Accessed November 18, 2020. https://medical.olympusamerica.com/sites/default/files/us/files/pdf/Whitepaper—Delays-in-Endoscope-Reprocessing-FINAL-APPROVED-single-page-version.pdf