
Small Problems with a Big Impact on Patient Safety
As reprocessing professionals, whether technician or leaders, there are common problems everyone can attest to. Ergonomic challenges, workflow issues, and process updates to name a few. Improving these can lead to a substantial return on investment, better engagement and a boost in compliance. However, there are sometimes unnoticed challenges within a department that may seem small or inconsequential that can contribute in big ways to patient safety.
In decontamination at the sink, there are several process tools that aid in both the cleaning and the monitoring process that we can often take for granted or may not get a second look in the hustle and bustle of daily volumes and throughput. With a closer look we can see that these quick fixes truly have a negative impact on the quality of care for the medical device and, ultimately, the patient.
Stickers
Adhesive temperature gauges and sink fill markers can provide a quick nod to compliance but frequently result in more harm than good in the long run. Adhesives used on stickers and tape require routine maintenance and replacement to ensure effectiveness and minimize its impact on microbial growth. Adhesive doesn’t hold up well during constant contact with water and can be found to peel and deteriorate overtime. As the stickers peel away the sticky residual is left behind acting as a bonding agent for bacteria, bioburden, and chemistry residuals present during the manual cleaning process. This can lead to biofilm formation within our sinks, or residual transference to our manual cleaned instrumentation.
Undetected Bioburden
If it isn’t clean, it isn’t sterile. Missed and undetected bioburden can be the source of a failed process. Visual inspection continues to gain traction as studies point to its benefits and the data 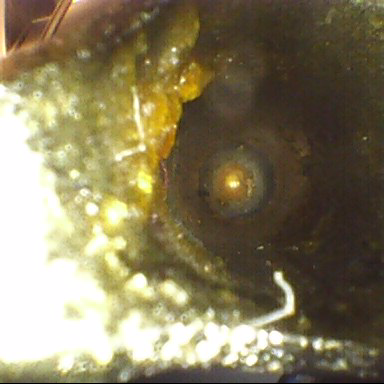 highlights the repercussions of left-behind bioburden. This leads to a broken-down process posing contamination risks to the patient, and inevitably instrument damage.
highlights the repercussions of left-behind bioburden. This leads to a broken-down process posing contamination risks to the patient, and inevitably instrument damage.
Some bioburden is detectable with the human eye; missed staples and/or bone fragments might be examples. When departments lack adequate lighting, however, even this bioburden can be missed without adequate lighting.
Bioburden goes beyond what the human eye alone can detect and without the right equipment, including lighting and visual enhancement tools, undetected bioburden can make its way through the cleaning process and beyond, leaving risk, corrosion and potential infection in its wake.
Leak Testing
Leak testing is a critical step in the manual reprocessing of flexible endoscopes. This test is the early indicator of scope damage and can also clue into the potential location of the damage. Leaks can occur at various points of the scope and can often be hard to identify or detect without proper visual accessories and minimum leak testing time requirements can lead to misinterpretation of leak test results.
test results.
A misinterpreted leak test whether, a missed fail or false fail, can impact patient safety in various ways including the risk of using a damaged scope, or eliminating inventory due to falsely identified issue that now results in insufficient procedural supply for patient volume.
Lighting is a critical factor to proper leak testing results. Ensure your department is equipped with proper overhead and possible in-basin lighting to assist in this QC step.
Water Temperature & Chemistry Dosing
Water temperature is another important consideration in the manual cleaning process. Temperature ranges aren’t just for optimal enzymatic performance but also for the prevention of blood coagulation. It is understood that blood can begin to coagulate at temperatures at or exceeding 113* F. Additionally, enzymes are activated, and their effectiveness can be based on optimal water temperatures. Too cool and the enzyme activation can be slow and “sluggish”, while too hot the enzymes can be extirpated altogether.
In addition, too little or too much cleaning chemistry can also have a negative impact on the cleaning process. Too little and organic soil isn’t effectively broken down. Too much and we have high foaming concentration in our cleaning solutions.
Without consistent, easy to read temperature and chemistry dosing monitors, the risk of inconsistency resulting in varied levels of cleanliness and cleaning effectiveness increases.
Electric requirements and power considerations
Powered equipment, such as height adjustable lifters, automated flushing, dosing pumps, and leak testers all have one thing in common; they require a power supply. This can cause challenges when implementing upgrades and updating workflows, as current electrical outlet locations may be sparse or non-existent in work areas, especially within decontamination.
Attempting to remedy electrical needs with power strips can be an accident waiting to happen. Shorted fuses or overdrawn power strips can be a fire hazard or electrical shock risk to staff. Long-term fixes are a necessity when electrical or power is being discussed.
Small issues often seem like a low priority, until we look at the risks associated with their quick fixes. Knowing the repercussions when work environments are not fully functional can aid in guiding how and what gets addressed as departments continuously improve their operations. Attention to detail is a prominent characteristic in the world of reprocessing, and when it’s applied to both our instruments and our processing tools staff and patients benefit from it.
Resources:
https://www.hpnonline.com/sterile-processing/article/13001537/keeping-murphys-law-out-of-the-spd
Are you looking for ideas to improve your department, and need a free CE? Check out our Ergonomics: Its Place in Strategic Planning Program today for other key areas to consider upgrading in your department.






