Starting your department on the road to ST91 compliance
Some of the most common minimally invasive procedures in the U.S. are endoscopy procedures, making endoscope reprocessing crucial to patient safety (ProMedica.org). Although common, these procedures do not come without risk; endoscopes are extremely intricate and advanced medical instruments, making them hard to clean by nature. “Contaminated endoscopes are the medical devices frequently associated with outbreaks of health care-associated infections” (NCBI.gov).
Patient safety, research, and process advancement continue to be a top priority for gastroenterology and sterile processing departments across the country. On March 3, 2022, AAMI released the updated ANSI/AAMI ST91:2021 Flexible and semi-rigid endoscope processing in health care facilities (AAMI.org). These guidelines were presented to help GI and central sterile departments create processes and procedures that reflect the latest research and advancement in endoscope reprocessing.
Amid nationwide staff shortages, leaders in gastroenterology and sterile processing department’s are more overwhelmed than ever. Implementing the latest ST91 updates into your department may seem like a daunting task that could include significant time, effort, or renovation costs, but getting started is easier than it looks.
Here are just 6 impactful improvements to help your department begin the journey towards ST91 compliance.
1. Implement height-adjustable equipment
Staff ergonomics continues to be an important factor in creating compliant departments that also reduce unnecessary costs and pain, like in workers compensation claims or days away from work. Consider making investments towards more ergonomic equipment when possible.
“Sinks, counters, and work surfaces should be:
- height-adjustable or positioned at heights that accommodate the average height of employees and the tasks to be performed at each location; and
- sufficient to accommodate the endoscope length”
Section 4.2.1 – General Considerations; ANSI/AAMIST91:2021
“Sinks should be height-adjustable so that personnel do not have to bend over to clean endoscopes. An ideal decontamination sink is height-adjustable, approximately 36 inches (91 centimeters [cm]) from the floor.”
Section 4.3.2 – Sinks and accessories; ANSI/AAMIST91:2021
2. Change the way you think about sink design
Sink design is more than height-adjustment. A properly designed sink that meets both compliance standards and department restrictions can also reduce device damage or repair costs. Basin sizes are one design element that can impact scope reprocessing, such as in total immersion in detergents or tight coiling.
“Sinks should be deep enough to allow complete immersion of the endoscope to minimize aerosolization. The size of the sink should be adequate (i.e., a minimum  of 16 inches x 30 inches) to ensure that the endoscope can be positioned without tight coiling and 8 to 10 inches (20 to 25 cm) deep, enabling a person of average size to work comfortably without undue strain on the back.”
of 16 inches x 30 inches) to ensure that the endoscope can be positioned without tight coiling and 8 to 10 inches (20 to 25 cm) deep, enabling a person of average size to work comfortably without undue strain on the back.”
Section 4.3.2 – Sinks and Accessories; ANSI/AAMIST91:2021
It may be prudent to invest in a custom sink design, or partner with a vendor who can provide consultations of challenging decontamination areas.
3. Implement a borescope to inspect high-risk endoscopes
If your department reprocesses high-risk endoscopes, now might be the time to evaluate a borescope program. While borescope programs can be complicated or expensive to implement, it may identify other areas of improvement for a department, like a lapse in manual cleaning or damages that occur during transport.
“High-risk endoscopes (e.g., duodenoscopes, linear ultrasound (EUS) endoscopes, bronchoscopes, endobronchial ultrasound (EBUS) endoscopes, ureteroscopes, cystoscopes, and as determined by the facility) shall be evaluated with cleaning verification tests after each use.”
Section 7.8.4 Cleaning Verification; ANSI/AAMIST91:2021
“Visual inspection alone is not able to determine if the reduction in clinical soil is sufficient for an effective cleaning result but is critical to determine any defects or potential damage to the endoscope.”
“Visual inspection can include the use of borescopes to inspect the inner channels/lumens present in many flexible endoscopes.”
Section 13.5.1 General Considerations; ANSI/AAMIST91:2021
Looking to implement a borescope program? Download our free checklist to ensure you’re considering all relevant areas impacted by integrating this technology into your department.
4. Implement lighted magnification for inspection
As important as internal inspection, having the proper illumination throughout a department can aid in assessing all endoscope varieties. Elevator channels and distal tips need particular attention for gross soil or other bodily substances.
“Lighted magnification should be used to inspect endoscopes and accessories for external cleanliness and damage.”
“According to the FDA/CDC, it is recommended that areas of the endoscope at the distal end be inspected using at least 5x magnification, 10x magnification for duodenoscopes.”
Section 7.8.2 Visual Inspection; ANSI/AAMIST91:2021
5. Enhance manual cleaning procedures
Manual cleaning is the foundation to effective high-level disinfection and sterilization outcomes. Any residual bodily fluids or debris can impact steps further along in endoscope reprocessing.
Automated flushing devices are one option to ensure endoscope channels are being flushed according to their manufacturer’s IFU while removing the variability of either manual methods, or between different practices among staff.
“Flush all channels according to the endoscope manufacturer’s written IFU.”
Section 7.6 Manual Cleaning Steps; ANSI/AAMIST91:2021
6. Better anti-fatigue mats
Keeping a clean work environment in endoscope reprocessing areas is critical. Ensure that high-touch items, such as anti-fatigue mats, are chosen that can be easily cleaned and maintained. Anti-fatigue mats also add further ergonomic benefits.
“Anti-fatigue mats should be:
- used in areas where prolonged standing is required;
- and constructed of materials capable of withstanding frequent cleaning”
Section 4.2.1 General Considerations; ANSI/AAMIST91:2021
Looking to implement any of these ideas or others for your endoscope reprocessing area? Pure Processing representatives are ready to learn about your space and provide guidance on potential reprocessing solutions. Contact us here to learn more.
Works Cited
ANSI/AAMI ST91:2021.
“Endoscopy.” ProMedica, https://www.promedica.org/services-and-conditions/endoscopy
Kovaleva, Julia, et al. “Transmission of Infection by Flexible Gastrointestinal Endoscopy and Bronchoscopy.” Clinical Microbiology Reviews, American Society for Microbiology, Apr. 2013, https://www.ncbi.nlm.nih.gov/pmc/articles/
“ST91: Endoscope Processing in Healthcare Facilities.” Default, https://www.aami.org/ST91.





 This surprise visit can create a sense of chaos or unease in the minds of some sterile processing and GI managers. Even a single observation can lead to a request for improvement (RFI). But by understanding some core fundamentals, any healthcare facility can be prepared for their next visit.
This surprise visit can create a sense of chaos or unease in the minds of some sterile processing and GI managers. Even a single observation can lead to a request for improvement (RFI). But by understanding some core fundamentals, any healthcare facility can be prepared for their next visit. of 16 inches x 30 inches) to ensure that the endoscope can be positioned without tight coiling and 8 to 10 inches (20 to 25 cm) deep, enabling a person of average size to work comfortably without undue strain on the back.”
of 16 inches x 30 inches) to ensure that the endoscope can be positioned without tight coiling and 8 to 10 inches (20 to 25 cm) deep, enabling a person of average size to work comfortably without undue strain on the back.”

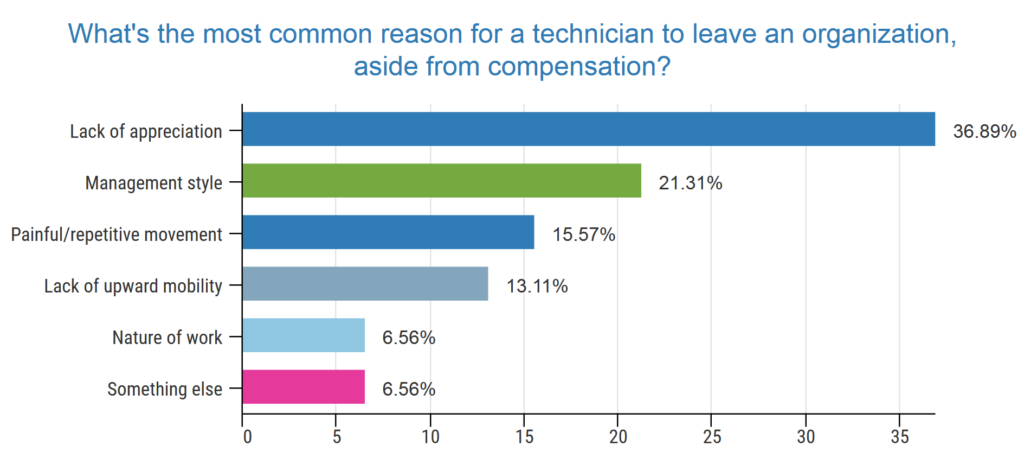
 certifications. Many organizations are screening applicants without certain certifications, but not adjusting compensation for those that do hold them. As states throughout the country mandate certain certifications, organizations may be finding it easier to justify as a “ticket to ride.”
certifications. Many organizations are screening applicants without certain certifications, but not adjusting compensation for those that do hold them. As states throughout the country mandate certain certifications, organizations may be finding it easier to justify as a “ticket to ride.”

 For example, consider the cleaning instructions for Zimmer orthopedic manual surgical devices (for hips, knees, trauma and extremities)1. The instructions list three separate options for cleaning and disinfection, ranging from rigorous manual to completely automated. While rigorous manual cleaning can take up to 45-50 minutes as prescribed by the IFU, sterile processing technicians only save about 10 minutes using a completely automated method using an automated washer and disinfector cycle.
For example, consider the cleaning instructions for Zimmer orthopedic manual surgical devices (for hips, knees, trauma and extremities)1. The instructions list three separate options for cleaning and disinfection, ranging from rigorous manual to completely automated. While rigorous manual cleaning can take up to 45-50 minutes as prescribed by the IFU, sterile processing technicians only save about 10 minutes using a completely automated method using an automated washer and disinfector cycle.






 Specifically, for lumened devices, AORN states:
Specifically, for lumened devices, AORN states:
