Sterile processing departments are evolving fast. With increasing instrumentation complexity, high surgical volumes, and stricter compliance requirements, the demand for technicians is growing by the day.
While traditional workstation setups offer a solid starting point, they’re often not equipped to handle today’s specialized process requirements. That’s why departments are turning to specialty sterile processing workstations, purpose-driven setups built to solve specific challenges within SPDs.
Here are 7 areas in prep and pack where specialty workstations can solve large problems or improve outcomes for sterile processing.
Consolidating Workflows with Wrap Inspection Spaces
The Problem: Frequent rewraps, overlooked damage on instrumentation and wrap, and crisscrossing patterns of workflow make wrapping spaces inefficient and ineffective.
The Solution: Specialty sterile processing workstations for centralizing sterilization wrap, lighting, indicators, and inspection tools into a single area. Built-in tabletop lights aid in wrap inspection before use. Technicians can better maintain wrap integrity, catch small tears or punctures, and streamline the entire packaging process.
Ample Space at Robotic Packing Stations
The Problem: Robotic arms and instrumentation require unique care and more space, but many general-purpose workstations fall short.
The Solution: Dedicated robotics sterile processing workstations provide ample surface area, organized access to heat sealers and robotic peel pouches, and integrated lighting—making it easier to handle robotic instruments without clutter or compliance issues. Only 1-2 larger-sized tables may suffice to keep up with robotic packaging needs.
Low-Temp Sterilization Compliance Made Simple
The Problem: Different sterilization methods require varying IFUs, indicators, and accessories, often leading to mistakes or delays.
The Solution: Low-temperature sterilization-focused sterile processing workstations position scales, tape dispensers, indicator storage, and computer systems in a more optimized location to improve accuracy and ensure AAMI and AORN compliance with less backtracking.
Loaner Tray Oversight You Can See
The Problem: Managing incoming loaner trays can be chaotic, especially with limited documentation or inconsistent check-in processes.
The Solution: Specialized loaner tray workstations with built-in scales, lighting, and mounts for computers or cameras enable technicians to verify weight, inspect contents, and document everything at one centralized station. By removing loaners from the general assembly area, departments can reduce bottlenecks, avoid cross-contamination, and improve traceability.
Space for Quality Assurance Tasks
The Problem: Critical QA tasks like borescope inspections or cleaning verification like ATP and protein testing are often squeezed into already busy workspaces.
The Solution: Quality assurance workstations are designed to house testing equipment safely, provide power access, and offer ergonomic adjustability. This creates a dedicated, organized space for these essential functions, freeing up other prep & pack areas. Smaller tables with built-in electrical can fit into already tight areas while adding capacity.
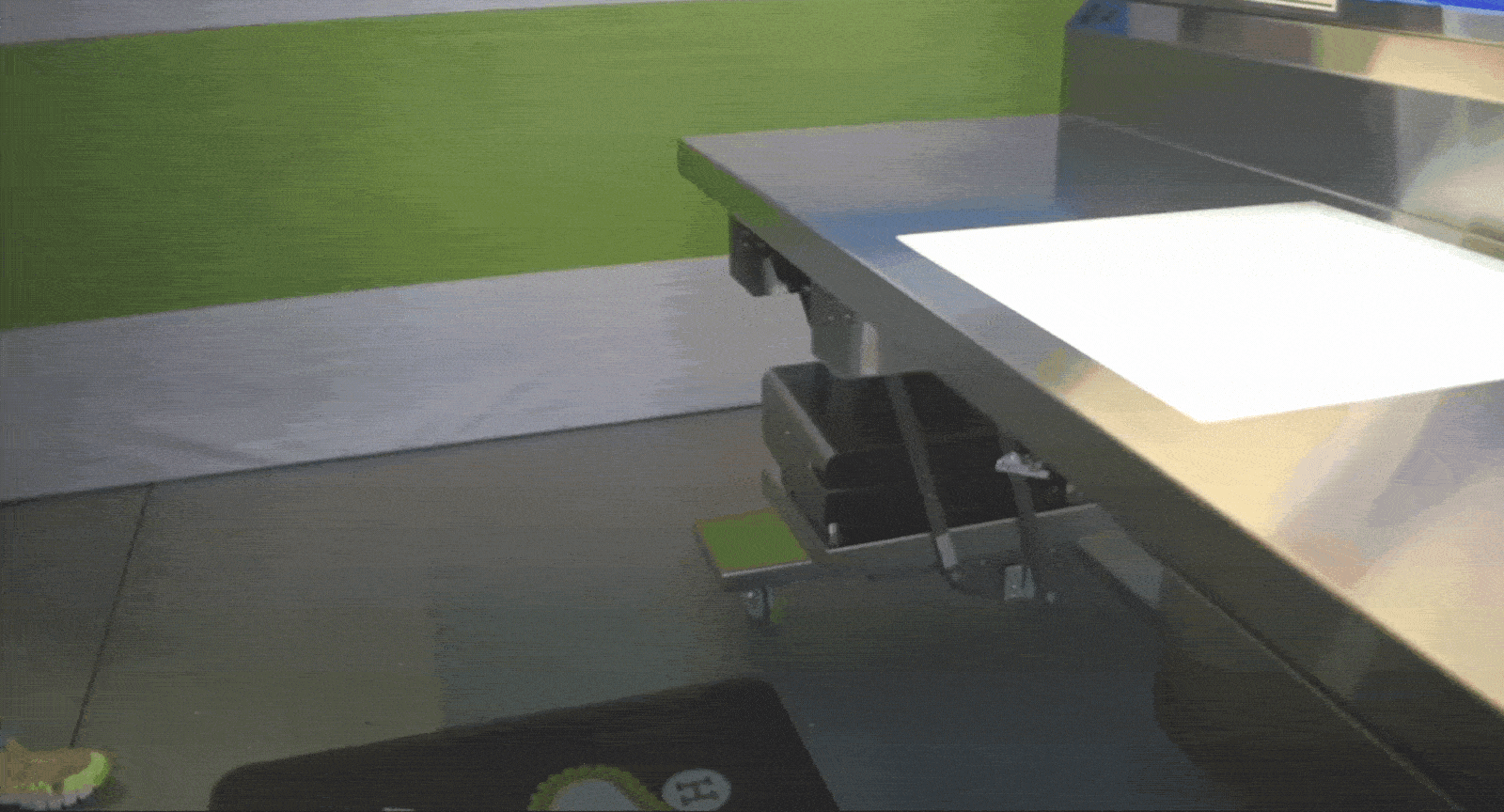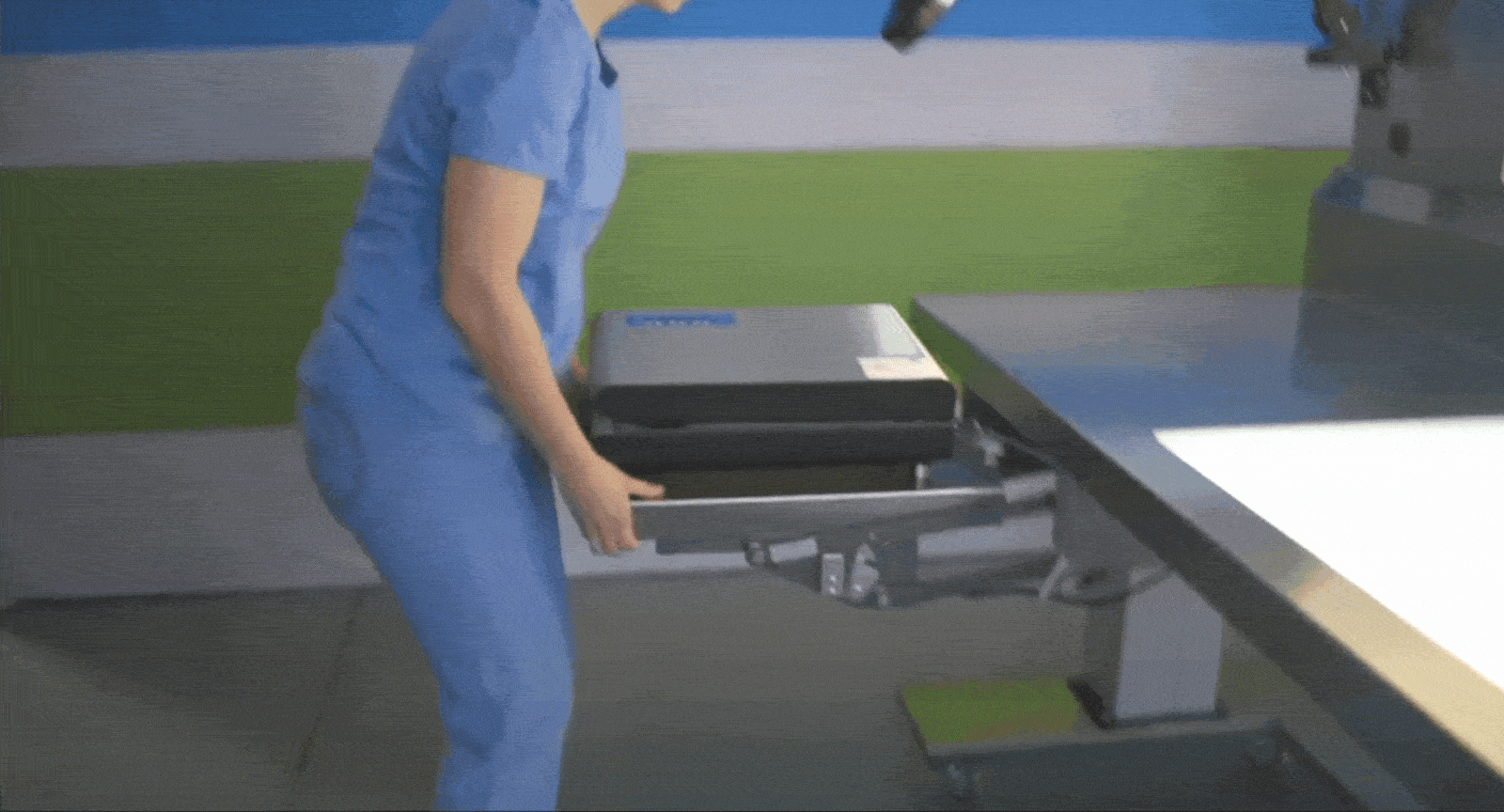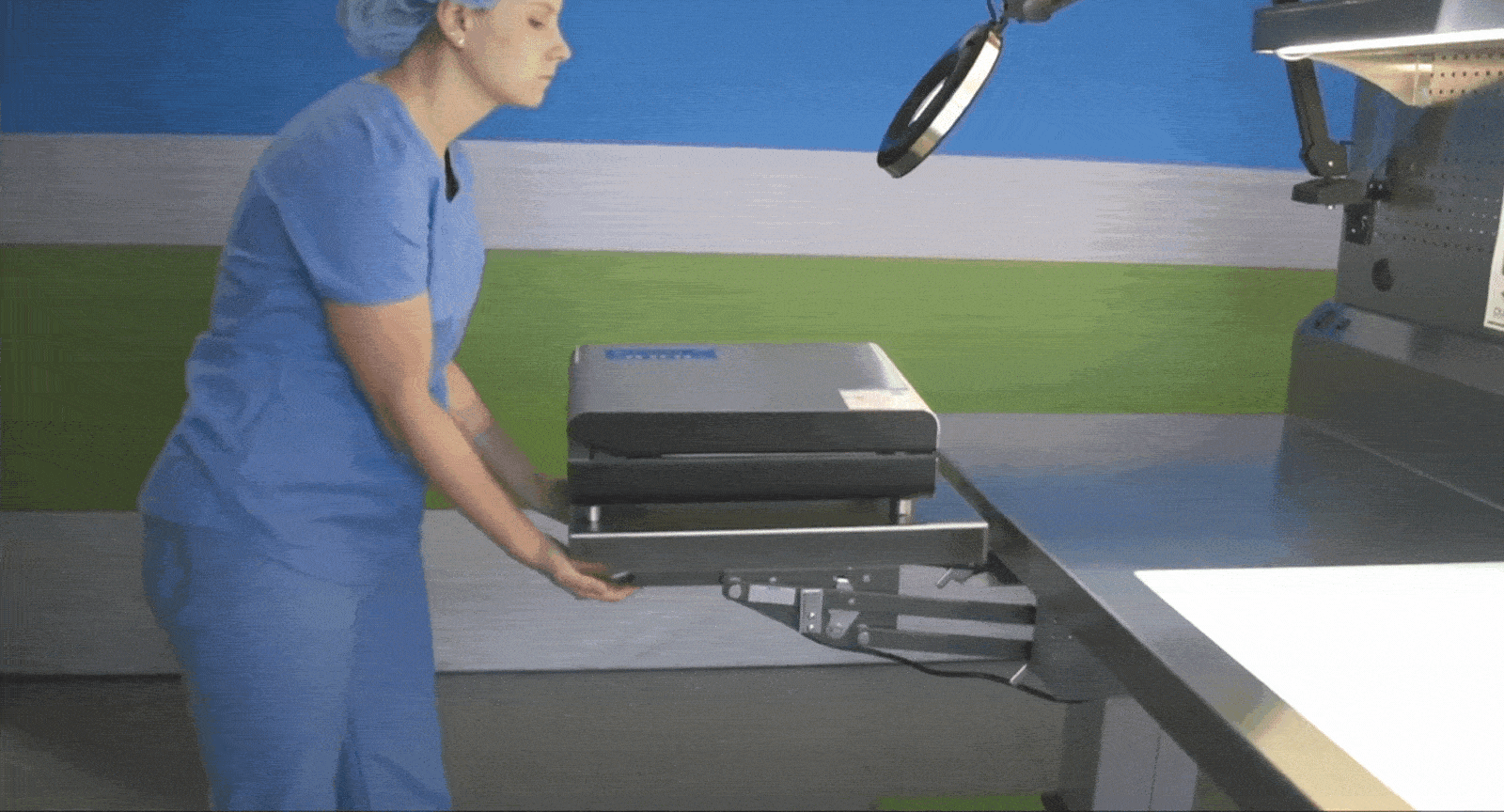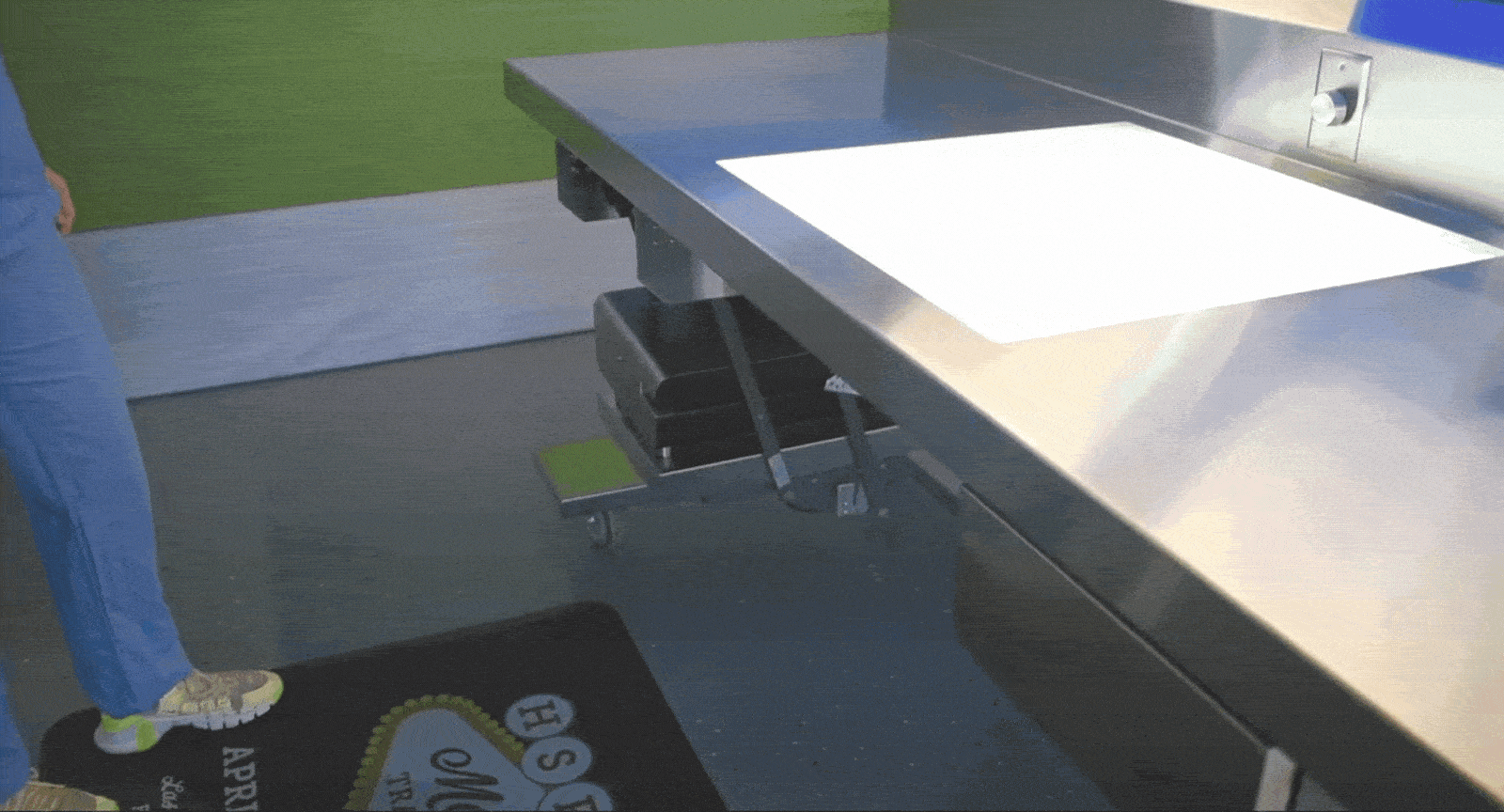
Tray Weighing and Compliance
The Problem: When scales are fixed in one location, it disrupts assembly workflows and adds unnecessary back-and-forth. Not having adequate scales at key areas also hinders loaner compliance.
The Solution: Mobile sterile processing tables with built-in scales allow departments to weigh trays exactly where it’s needed, ensuring they meet weight limits without compromising efficiency. Tables can fit snugly between packing stations, or at vendor drop-off points.
Compact Options for Tight Spaces
The Problem: Some departments simply don’t have the space to expand but still need to improve wrap inspection or add work surfaces.
The Solution: Miniature sterile processing workstations with integrated lighting and storage offer powerful functionality in a compact footprint. These stations can be moved, shared, or placed in smaller packaging zones to meet growing needs without construction budgets.
A New Approach to Sterile Processing Workstations
Specialty sterile processing workstations aren’t just about adding equipment, they’re about redefining how departments support their technicians and processes. Instead of forcing tasks into standard configurations, some workstations are designed around actual workflow needs. Departments need not replace their entire fleet of tables to see visible productivity and compliance gains!
By choosing the right configurations for robotic packaging, low-temp compliance, loaner tray handling, or QA testing, facilities can eliminate bottlenecks, improve safety, and raise the overall standard of care.
As sterile processing continues to evolve, one thing is clear: the most effective workstations are the ones that solve real problems.
Interested in learning about our solutions for sterile processing workstations & impact tables?
Contact us today to explore solutions tailored to your team’s needs.








 to instrument cleanliness, instrument accuracy and sterile barrier preparation. Having any of these tools separated from the other will lower completed inspection points while increasing your non-compliance variable. Consider staging all these tools together into comprehensive stations.
to instrument cleanliness, instrument accuracy and sterile barrier preparation. Having any of these tools separated from the other will lower completed inspection points while increasing your non-compliance variable. Consider staging all these tools together into comprehensive stations.

 departments optimize instrument sets, reducing excessive weight and improving overall workflow. By ensuring trays meet industry standards, sterile processing helps OR teams handle instruments safely and efficiently, preventing unnecessary strain or injuries.
departments optimize instrument sets, reducing excessive weight and improving overall workflow. By ensuring trays meet industry standards, sterile processing helps OR teams handle instruments safely and efficiently, preventing unnecessary strain or injuries.