Properly reprocessing flexible and rigid endoscopes is a critical element of the healthcare infection control process, and following best practices is pivotal. Scott R. Lucas, PhD, PE, program manager, engineering, of ECRI Institute’s AFI Group says, “Hospitals should be diligent in addressing risks related to endoscope reprocessing before a contamination—or even worse, a patient infection—occurs.” In 2010, the ECRI Institute named cross contamination from flexible endoscopes as the top medical technology hazards.
Flexible endoscopy, while common, is subject to high infection control risk. Infection can occur in three ways:1
- Patient to patient
- Environment to patient
- Organisms within the patient’s body
Clinical studies have found reusable endoscope infection risk to be linked to micro-organisms clinging to endoscope biomaterial surfaces. The process forms biofilms that often remain wet after processing and lead to a contamination risk.“The formation of endoscopic biofilm during clinical practice can be related to reuse of detergent, manual cleaning, and incomplete drying of processed endoscopes. Developed biofilms protect the micro-organisms from exposure to detergents and germicides, which increase the likelihood of survival through a decontamination process.”2
Following the guidelines and the manufacturer’s instructions for use (IFU) during pre-cleaning of the scope is important. This process will remove most of the bioburden after the procedure. Thorough flushing and brushing before any disinfection procedure is necessary to complete the job.
It’s a task that your reprocessing department performs often, considering that a single scope averages 300 to 1,200 uses per year.
Many departments have slightly different manual cleaning protocols for flushing because the differences in flexible and rigid scopes. It is, however, one of the most critical steps to ensure complete removal of bioburden prior to high level disinfection. It’s helpful if your department has one manual cleaning tool that can meet IFUs for your full scope inventory.
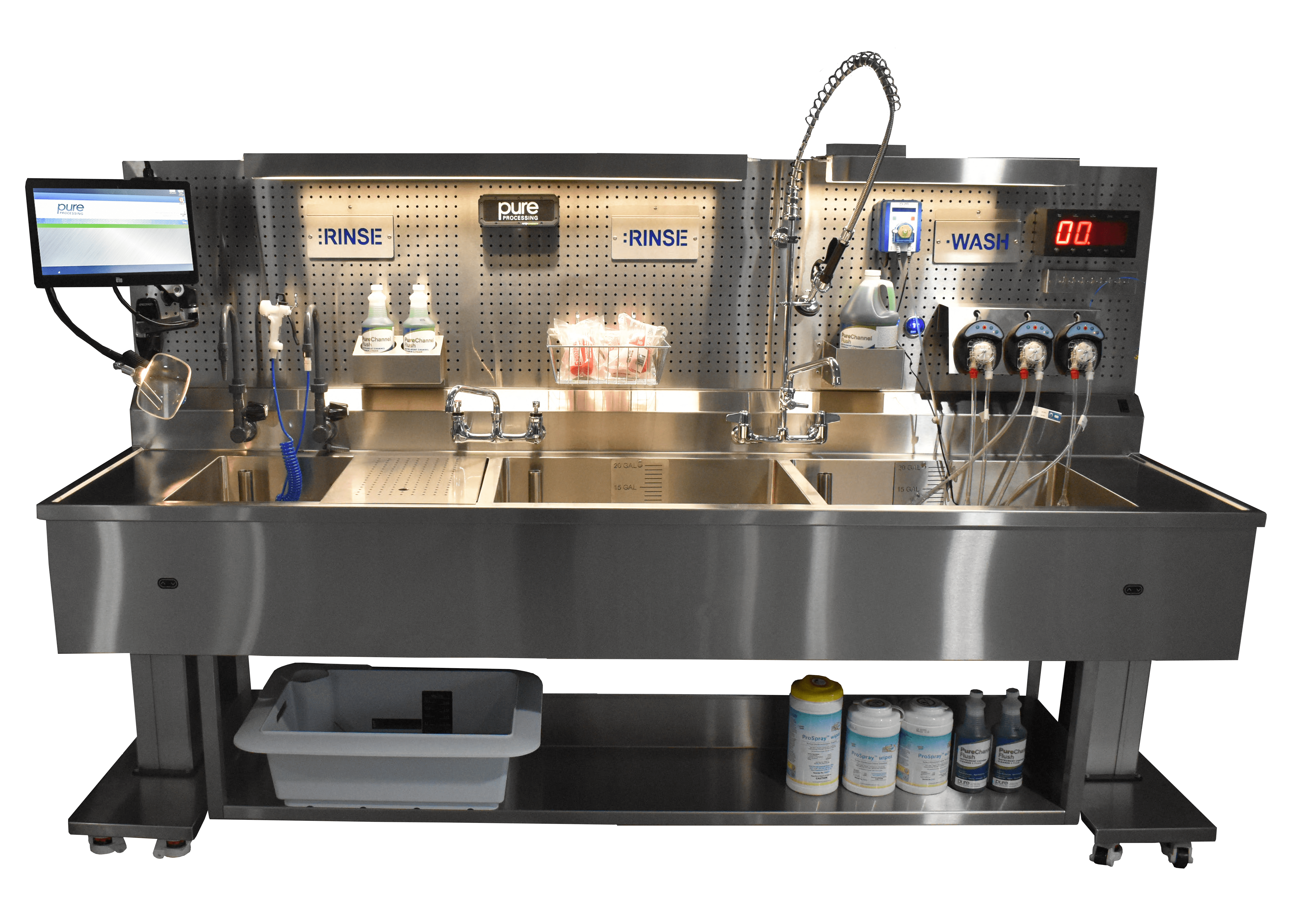
Reducing infection risks with proper scope cleaning protocols
Rigid and flexible endoscopes should be wiped down immediately after use and kept moist. Then the scopes are placed in an enzymatic detergent solution.During manual cleaning, a nurse should brush and aspirate all channels, flushing the channels with detergent.
Perform these steps even on channels not used during the endoscopic procedure. Clean removable parts separately.
Manual cleaning should include brushing and flushing channels and ports according to the manufacturer’s IFU. Using warm tap water for deep cleaning and a disinfectant-detergent solution is standard. Be sure to flush away all disinfectants after cleaning, as they can cause deterioration to delicate instrumentation over time. These areas can become susceptible to hidden microorganisms and pathogens that can infect the next patient. Staff must handle endoscopes with care to avoid excessive coiling, twisting or buckling.

Cleaning will require multiple steps and use up to two sinks (preferably three):
- Sink 1: Soak in cleaning solution
- Sink 2: Rinse
- Sink 3: Treated water rinse
Determining the best endoscope flushing solution for your department
Staff must be as efficient as possible to process a high volume of endoscopes. Staff must also be meticulous in performing scope processing tasks to ensure compliance to cleaning protocols. That’s when having one solution to manually clean different types of scopes and meets compliance, makes a difference. The manual cleaning steps prior to high level disinfection must be thorough and meet IFU and guidelines.
If you don’t have a good endoscope flushing pump in your department, look for these features:
- Works with flexible and rigid scopes
- Has the proper adapters for flexible and rigid scopes
- Reduces exposure to chemicals
- Reduces ergonomic impacts to technicians
- Offers an automatic flushing option to run independently
- A pressure relief valve to protect delicate scope channels is compatible with multiple scope channel diameters and sizes
- Can flush cleaning and rinse solutions
Identifying a solution to best fit your department needs, follow IFU, and protect staff from injury is ideal. A proper manual cleaning flushing device should do all of the above and can enable your team to manage a high volume of endoscope processing while achieving staff and, ultimately, patient safety.
Learn more about the FlexiPump’s endoscopic cleaning capabilities, how it can improve staff ergonomics, and automate manual flushing.
Have a problem you’d like help tackling? Let us know!
Would you like to earn free CE’s? Read these related endoscopy blogs and take the quizzes for 0.5 CE each!
Endoscope Manual Cleaning: The Challenges that Impact Our Effectiveness
4 Process Trends in GI and SPD & What You Need to Know
References:
- Crowson, Carol (2000). Cleaning and disinfecting flexible endoscopes. Nursing Times.
Vol: 96, issue: 38. - Omidbakhsh, N. et al (2021). Flexible gastrointestinal endoscope processing challenges, current issues and future perspectives. The Journal of Hospital Infection. Volume 110, P133-138, April 01, 2021.
- ANSI/AAMI ST91:2021: ANSI AAMI ST91 | Flexible and semi-rigid endoscope processing | AAMI
- ANSI/AAMI ST79:2017: ANSI AAMI ST79 2017 | AAMI





 IFUs are “written recommendations provided by the manufacturer that provide instructions for operation and safe and effective use of its device. …The device manufacturer is responsible for ensuring that the device can be effectively cleaned and sterilized with the means and methods available in health care facilities. Sterilization validation of a device requires microbiological, engineering, toxicological, and sometimes clinical evaluations of the device, which are beyond the abilities of most health care facilities. To ensure patient safety, a reusable device needs to be capable of being thoroughly cleaned and sterilized. The device labeling describes specific methods of cleaning and sterilization that have been validated by the manufacturer.”
IFUs are “written recommendations provided by the manufacturer that provide instructions for operation and safe and effective use of its device. …The device manufacturer is responsible for ensuring that the device can be effectively cleaned and sterilized with the means and methods available in health care facilities. Sterilization validation of a device requires microbiological, engineering, toxicological, and sometimes clinical evaluations of the device, which are beyond the abilities of most health care facilities. To ensure patient safety, a reusable device needs to be capable of being thoroughly cleaned and sterilized. The device labeling describes specific methods of cleaning and sterilization that have been validated by the manufacturer.”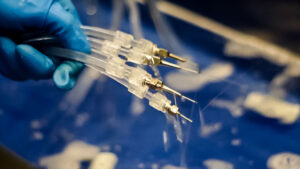 IFUs address several areas of concern. Many instruments or devices have numerous parts that must be disassembled for proper reprocessing. Understanding how those intricate parts work, the design elements, and possibly overlooked areas for cleaning, are essential to ensuring that a device is sterilized. Cleaning solution IFUs are another area that need to be taken into consideration. Proper dilution, concentration, temperature and contact time are vital for ensuring proper decontamination and sterilization. Additionally, some instruments and devices cannot be treated with specific solutions. Following manufacturers’ IFU will help prevent unnecessary damage the instrument. IFUs for reprocessing equipment are equally important. Without proper knowledge of the tools used to reprocess the instruments, effective reprocessing cannot be achieved.
IFUs address several areas of concern. Many instruments or devices have numerous parts that must be disassembled for proper reprocessing. Understanding how those intricate parts work, the design elements, and possibly overlooked areas for cleaning, are essential to ensuring that a device is sterilized. Cleaning solution IFUs are another area that need to be taken into consideration. Proper dilution, concentration, temperature and contact time are vital for ensuring proper decontamination and sterilization. Additionally, some instruments and devices cannot be treated with specific solutions. Following manufacturers’ IFU will help prevent unnecessary damage the instrument. IFUs for reprocessing equipment are equally important. Without proper knowledge of the tools used to reprocess the instruments, effective reprocessing cannot be achieved.
 Keeping items easily within reach is key to reducing musculoskeletal disorders from repeated reaching. By employing an integrated
Keeping items easily within reach is key to reducing musculoskeletal disorders from repeated reaching. By employing an integrated 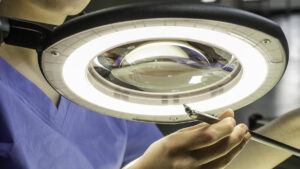 In 2013, researchers found that by integrating a few simple ergonomic changes into a department’s design can help improve staff safety. In one department, “a lack of space in the packing area,” led to “…undesirable twists when moving around equipment.”4 By leaving “enough space to establish safe and sound work processes,” the department’s workflow improved.
In 2013, researchers found that by integrating a few simple ergonomic changes into a department’s design can help improve staff safety. In one department, “a lack of space in the packing area,” led to “…undesirable twists when moving around equipment.”4 By leaving “enough space to establish safe and sound work processes,” the department’s workflow improved.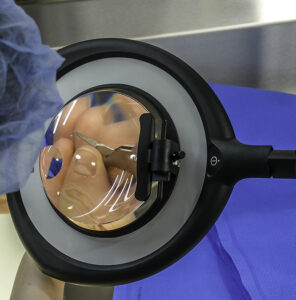 Many instruments have very small pieces and tips that look quite similar to the naked eye. It is even more difficult to notice these subtle differences under inadequate lighting. A 2019 study regarding errors in packaging surgical instruments found that “personnel error is the primary reason for packaging errors. Central sterile supply department (CSSD) staff members are not familiar with the clinical utilization of surgical instruments, and therefore it was hard for them to distinguish between instruments with minor differences.”5
Many instruments have very small pieces and tips that look quite similar to the naked eye. It is even more difficult to notice these subtle differences under inadequate lighting. A 2019 study regarding errors in packaging surgical instruments found that “personnel error is the primary reason for packaging errors. Central sterile supply department (CSSD) staff members are not familiar with the clinical utilization of surgical instruments, and therefore it was hard for them to distinguish between instruments with minor differences.”5 Additionally, when wrapping trays with woven and nonwoven sterile wraps, ANSI/AAMI ST79:2017, 9.5 states that departments must “inspect the wrap to ensure that it is free of defects that could have an adverse effect on the performance of the material.”
Additionally, when wrapping trays with woven and nonwoven sterile wraps, ANSI/AAMI ST79:2017, 9.5 states that departments must “inspect the wrap to ensure that it is free of defects that could have an adverse effect on the performance of the material.”




