Are Your Tube Sets Harboring Bacteria or Preventing IFU Compliance?
Sterile processing departments continue to invest in automated flushing systems capable of flushing virtually all reusable lumened devices to optimize efficiency and meet cleaning IFUs. That system often is the FlexiPump Independent Flushing System. It is a reliable piece of equipment to be sure. However, there is something you may not be doing that will compromise its cleaning and performance abilities. That something would be tube set maintenance.
Let’s take a closer look at FlexiPump tube sets and their proper maintenance needs, which can support the longevity of the system while ensuring your team meets instrument cleaning IFU requirements.
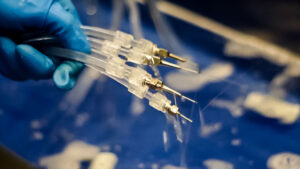
FlexiPump tube set styles and valves
The FlexiPump has three styles of tubing available:
• Two-lead for robotic instruments and other, dual channeled instruments
• Three-lead for SPD, GI use
• Five-lead for ocular, suction tip, or devices smaller than 2 mm in diameter
One of the best functions of the FlexiPump is the ability to use all the tubes at once to flush multiple devices at a time. That’s a huge win for departments short on time, staff, and resources.
Important components of the tubing are the 15 and 30 PSI valves. The purpose of these valves is to maintain even pressure to protect delicate internal lumens of instruments during the flushing process.
What happens without proper tube set care?
A few issues can occur if the FlexiPump tube set care recommendations are not followed. Lost of elasticity is one such issue. Tubing will naturally lose its elasticity through the course of use. Loss of elasticity makes it difficult for the pump to maintain proper flow rate. Flow rate is important to meet IFUs and perform effective device cleaning. This is one reason why changing out tube sets every month is necessary. FlexiPump tube sets include a 30-day TimeStrip to help technicians keep track of how long each tube set has been in use.
Infection prevention is also a high priority reason for following recommended tube set instructions. The whole purpose of reprocessing instruments is to remove infection risk for the next patient. Hard water deposits can build up inside the tubing and foster biofilm buildup. Proper tube set maintenance such as disinfecting and swapping out tube sets every month can help ensure you meet manual cleaning goals to prevent hospital-acquired infections.
Did you know tube sets have lubricant to support the pump’s motor function? The lubricant eventually wears away and causes unnecessary strain on the motor as the pump works harder to turn the tubing assembly. When you change FlexiPump tube sets to support infection prevention and avoid the loss of elasticity, you are also preserving the pump itself.
Proper tube set care safeguards pump longevity and proper instrument cleaning
Tube sets are a critical component of the FlexiPump to meet thorough instrument cleaning goals. Proper maintenance is actually quite easy and has a positive impact overall. Two specific activities should be in your maintenance schedule: disinfecting and changing tube sets.
1. Disinfecting FlexiPump tube sets
Disinfecting tube sets is a simple step and can be added as a routine activity with each shift change. FlexiPump tubes should be disinfected after every shift, or at a minimum of once a day. That will keep them clean and maintain the needed elasticity for best performance. ProSpray™ Surface Disinfectant (as per the IFUs) is recommended for decontamination. It does not contain bleach or alcohol. View the full instructions for disinfecting the tube set to follow IFUs.
2. Changing FlexiPump tube sets
It is ideal to change your FlexiPump tube sets each month. Doing so supports proper function of the device and infection prevention because tubes are changed before they dry out, lose elasticity, or harbor biofilm. Loss of elasticity can reduce flow rate and compromise your IFU compliance. Lubricant wears away in tube sets left too long on the pump. That results in more resistance and work for the pump and may reduce the pump’s lifetime. Watch our in-service video for instructions on how to change your FlexiPump tube sets.
Prevent biofilm buildup and pump damage
Proper maintenance of your FlexiPump tube sets will set your team up for successful infection prevention and staff efficiency. Simple efforts such as disinfecting after each shift, and replacing tube sets every 30 days require little time but offer the big results you need from a flushing system device.
Our service team is always ready to support your department with educational resources and in-service opportunities. Contact your sales representative for more information or call us at 877-718-6868 to speak with one of our service members. You can also visit our full interactive page here for more information on use and care of your FlexiPump, including video demos.
Learn more about the Pure Processing FlexiPump Independent Flushing System and how it can ensure your surgical devices are cleaned according to IFUs, save your team valuable time, and reduce the risk of repetitive motion injury.
Find more resources for FlexiPump™ Support | Pure Processing (pure-processing.com)




 Let’s try a football analogy. The vision aspect could be winning the vital rivalry game. The goals are small, achievable accomplishments toward the win. First downs, or a deep punt that puts the offense on its heels, and of course touchdowns and extra points, are important goals toward winning that rivalry game.
Let’s try a football analogy. The vision aspect could be winning the vital rivalry game. The goals are small, achievable accomplishments toward the win. First downs, or a deep punt that puts the offense on its heels, and of course touchdowns and extra points, are important goals toward winning that rivalry game.
 Creating a patient-first culture which incorporates employee psychological safety is important for teams to achieve success.
Creating a patient-first culture which incorporates employee psychological safety is important for teams to achieve success.


 cleaning all take up quite a bit of time. Now, imagine if you had to walk all over the department just to get tools and accessories. Or if the sinks were at awkward heights and did not match the user’s physical needs. Imagine having to stop and reposition repeatedly to accomplish tasks. That would all contribute to a rapidly diminishing productivity outcome. One that can be further reduced when technicians are in pain from working in unaccommodating conditions.
cleaning all take up quite a bit of time. Now, imagine if you had to walk all over the department just to get tools and accessories. Or if the sinks were at awkward heights and did not match the user’s physical needs. Imagine having to stop and reposition repeatedly to accomplish tasks. That would all contribute to a rapidly diminishing productivity outcome. One that can be further reduced when technicians are in pain from working in unaccommodating conditions. Carts are often a necessity for central sterile departments. Their use for soaking and transport may seem to be rather simple, but don’t let that fool you.
Carts are often a necessity for central sterile departments. Their use for soaking and transport may seem to be rather simple, but don’t let that fool you. Peak reprocessing hours can create a bottleneck for productivity. And we all know the last thing we want to do is hold up the operating room or our decontamination techs. Creating and maintaining flexible workstations can solve the bottleneck and help your team accomplish more with less.
Peak reprocessing hours can create a bottleneck for productivity. And we all know the last thing we want to do is hold up the operating room or our decontamination techs. Creating and maintaining flexible workstations can solve the bottleneck and help your team accomplish more with less. Flushing is a huge productivity block. Flushing one instrument at a time is something more and more departments are moving away from, thanks to new technologies that allow for multiple instruments to be flushed at the same time. That saves technicians time, enables them to perform additional tasks, and ensures IFU compliance.
Flushing is a huge productivity block. Flushing one instrument at a time is something more and more departments are moving away from, thanks to new technologies that allow for multiple instruments to be flushed at the same time. That saves technicians time, enables them to perform additional tasks, and ensures IFU compliance.
 syringes. That’s because syringes cannot be calibrated to consistently perform for the user. They are a manual tool without presets for pressure or volume, and that leads to inconsistency. Syringes cannot indicate when manual cleaning and flushing steps are complete or thorough.
syringes. That’s because syringes cannot be calibrated to consistently perform for the user. They are a manual tool without presets for pressure or volume, and that leads to inconsistency. Syringes cannot indicate when manual cleaning and flushing steps are complete or thorough. surface up while washing items in the sink.” Technicians often find themselves reaching into deep sink basins to properly clean instruments in decontamination. While deep sinks may be required for heavy soaking or larger trays, they are not conducive for most manual cleaning tasks. Prolonged bending can lead to lower back, shoulder, and neck pain over time, creating more serious issues in the future. Incorporating a
surface up while washing items in the sink.” Technicians often find themselves reaching into deep sink basins to properly clean instruments in decontamination. While deep sinks may be required for heavy soaking or larger trays, they are not conducive for most manual cleaning tasks. Prolonged bending can lead to lower back, shoulder, and neck pain over time, creating more serious issues in the future. Incorporating a  and assembly tables often come with many accessories and mounted items such as monitors, shelves, or powered equipment. These accessories, used often and throughout reprocessing tasks, should be positioned on equipment to reduce reaching over shoulder height, and avoid flexion pain for employees. Height-adjustable equipment that allows the entire station, including their many accessories, to adjust to the user’s most optimal height should be considered when designing tables and sinks.
and assembly tables often come with many accessories and mounted items such as monitors, shelves, or powered equipment. These accessories, used often and throughout reprocessing tasks, should be positioned on equipment to reduce reaching over shoulder height, and avoid flexion pain for employees. Height-adjustable equipment that allows the entire station, including their many accessories, to adjust to the user’s most optimal height should be considered when designing tables and sinks.