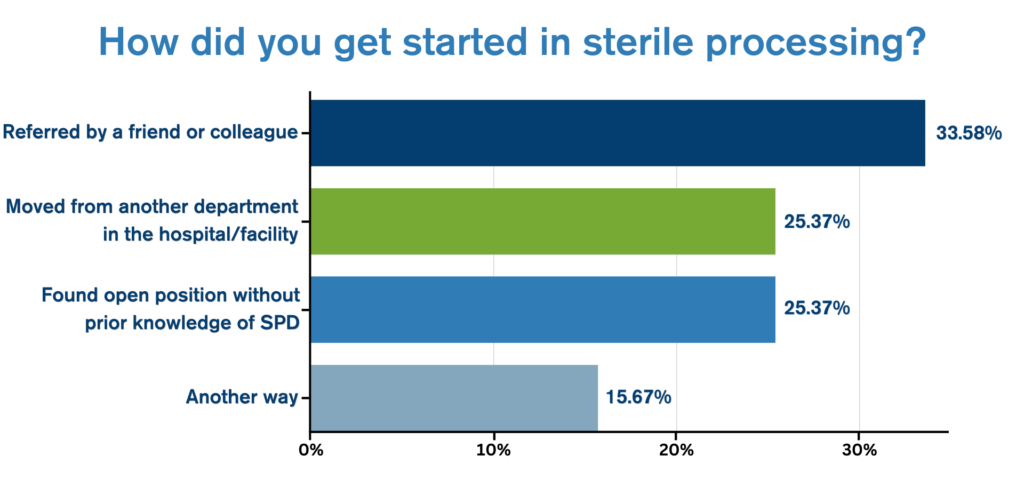
Endoscopy managers have a big job. Between staffing, training, and ensuring the needs of their department are met, there’s no shortage of challenges.
Endoscope reprocessing, too, has been under a microscope by infection control practitioners, directors, and surveyors for years, and with good reason. The number of endoscope adverse events in quarter 4 of 2022 reported by the Food and Drug Administration (FDA) in the MAUDE database is shown to be more than 10 times the number of adverse events in the same quarter in 2018 (6,161 versus 373). These adverse events have increased every quarter since quarter 2 of 2021. Search the MAUDE database, here.
But often the resources allotted to endoscopy managers do not match the rising complexity in devices and their IFU. Gastroenterology departments differ in notable ways from their instrument reprocessing counterparts in sterile processing. It’s important to evaluate these unique challenges to ensure they’re addressed with the finest accuracy possible in a gastroenterology setting.
Reprocessing Rooms
Gastroenterology departments often suffer from small, cramped reprocessing rooms. Nearly every regulatory body or accrediting organization seeks to establish “one-way” directional flow separating clean workspaces from dirty. Limited space makes compliance incredibly challenging for endoscope reprocessors and managers to accommodate. Space is often a major issue for endoscopy managers, and there’s no easy way to remedy it.
General lack of space also means staging space is severely limited, which can lead to other problems, such as stacking scopes. Stacking scopes creates hazards for both infection control and the device itself. Many gastroenterology managers resort to vertical staging as their only compact solution.
When reprocessing spaces are too small for the demands of a facility, equipment needs also suffer. Many reprocessing sinks are not only too small, but often too deep to properly coil endoscopes, and feature few of the necessary tools needed. Lighting plays a critical role in cleaning verification, and yet most sinks do not have the room or space to add overhead lighting or task lights for focused inspection.
ANSI/AAMI ST91:2021 provides great guidance regarding lighting recommendations:
“Generally, all functions performed within a processing area require detailed and accurate inspection. Ancillary lighting should be considered for areas where instruments are manually cleaned and inspected. Lighting fixtures should be selected and mounted in positions that focus the light in front of the employee so that they are not working in their own shadows.” 4.3.8 Lighting
Space is such a pervasive challenge in gastroenterology, it creates challenges for handwashing sink compliance, emergency eye wash stations, worker mobility, and more. For guidance on making the most out of your space, check out our Study Guide of Space blog post for ideas.
High-Level Disinfection
Most endoscopy departments rely on high-level disinfection as the final cleaning treatment before storage. The recent change in ANSI/AAMI ST91:2021 has spurred significant discussion around sterilizing high-risk endoscopes. Both ethylene oxide and hydrogen peroxide methods are available, however, drawbacks follow, including incompatibility with scope models, longer processing times, and potential for carcinogenicity.
While ANSI/AAMI ST91:2021 doesn’t yet mandate sterilization for high-risk endoscopes, the change is likely inevitable, and will create challenges regarding the integration of sterilization technologies into departments, and ultimately increase the cost to process scopes. Space will continue to be a struggle, and entire department workflows may need to be evaluated. Training will need to be added and increased, as well. Departments may also find themselves re-evaluating their scope inventories, and investing money into new endoscope models, such as those with disposable components.
Gastroenterology departments have many challenges to consider tackling in order to achieve excellence in endoscope reprocessing compliance. It is also important to evaluate gastroenterology sinks in a separate lens than that of sterile processing to ensure they’re implementing the most impactful solutions in their departments.
Looking for guidance on a potential endoscope reprocessing sink project? Download our free Endoscope Reprocessing Sink Checklist, with 16 key considerations for selecting the right solution, including workflow compliance, space considerations, and more.




 Many sterile processing department managers know how it feels to learn that your facility is about to undergo a Joint Commission (JC) survey; even for the most prepared departments, it can be anxiety-inducing.
Many sterile processing department managers know how it feels to learn that your facility is about to undergo a Joint Commission (JC) survey; even for the most prepared departments, it can be anxiety-inducing.
 well knowing how to readily access, retrieve, and present them, demonstrates that your department is well organized, and has done the work to ensure policies and procedures are established and accessible.
well knowing how to readily access, retrieve, and present them, demonstrates that your department is well organized, and has done the work to ensure policies and procedures are established and accessible.




 for those timers to be misplaced or forgotten about. This can result in wasted time, an inability to accurately time certain tasks, or insufficient cleaning time per a device’s IFU.
for those timers to be misplaced or forgotten about. This can result in wasted time, an inability to accurately time certain tasks, or insufficient cleaning time per a device’s IFU.