Voice of the Customer
In March, our Voice of the Customer (VOC) Committee gathered to discuss a topic that can play a critical role in sterile processing departments but often gets overshadowed: Data.

Jhmeid Billingslea
While it’s easy to acknowledge the benefits of collecting and using data to solve problems in sterile processing, the process can be a bit daunting. Where do you start if you want to start getting insights on your department through data?
We took the conversations from our VOC call and went a step further, collaborating with longtime VOC member, Jhmeid Billingslea of Advantage Support Services, to give readers some direction on beginning to collect and use data in their sterile processing departments.
Productivity
The first step towards becoming a data-driven department is honestly assessing exactly how productive your department is. If you’re perpetually behind on cases, there won’t be any time or energy available to begin taking on new initiatives. Struggling productivity can also make it feel like a department is understaffed, further worsening a team’s perceived bandwidth. A productive department is a proactive department, so tackling issues that reduce productivity, or implementing tools that can boost it, is important for departments to consider.
Unfortunately, recognizing productivity issues can be difficult without addressing them. If your department might be under performing, take these two basic steps
- Look at the total number of trays completed vs the number of people in your department.
- Assess the output of your highest-performing employees against the output of your team.
Determine how many trays your team completes per shift and compare it to the number completed by your high performers. This allows you to start identifying ways to optimize task completion across the department.
Quality assurance
The next step towards becoming a data-driven department is implementing quality assurance policies to ensure what your department produces is accurate, correct, and safe to use.
Rating carts coming into your department is a critical component to begin collecting data, and the benefit is two-fold:
- If every cart coming from the OR is assessed and rated by a member of your team, you now know when that cart first showed up to the department. This will help you determine how long it takes your department to fully reprocess it.
- Identifying and rating carts allows your SPD to clearly identify damages and concerns with point-of-use cleaning before any reprocessing is done. Reporting poor ratings and failures to the OR manager can help improve pre-cleaning practices before SPD receives an instrument, helping to reprocess it more efficiently.
Implementing proactive quality assurance measures before storage is also a big piece of the puzzle. This approach helps you identify deficiencies in your department and reduce the number of failed trays sent to the OR.
A couple of best practices to keep in mind:
- Importance of categorizing trays and tray complexity. Not all trays are equal; completing 10 basic trays may take the same amount of time as completing 3 complex ones. Gauging performance requires factoring in complexity, and planning must account for it. You gain a better understanding of how much time is spent on specific types of trays and add qualitative information to your data.
- Avoid opting for simple solutions to meet quality requirements. Once you’ve identified the most complex cases that have the highest likelihood for error, increase the required QA quota for them to learn more about how to improve on them.
With your department’s productivity level understood and QA procedures in place, it’s now time to start collecting and using data in your department. What information should you collect, and what hurdles might you encounter in the process?
Get your team on board
A significant challenge related to getting good data about your department’s performance is soliciting team involvement in data collection. Many see it as an additional, unnecessary step that only makes their lives more difficult. In reality, this could not be further from the truth.
By demonstrating a few points to your team, you can get on the same page about the usefulness of data in SPD:
- Insights from departmental performance can help you justify investments for more staff, new equipment, and training to help your team achieve their goals more easily.
- Individual performance metrics give managers objective information they can point to when looking to give raises or promotions, offering sterile processing professionals more opportunities to grow in the industry.
- Proactive quality assurance and cart ratings can help improve communication with the OR and solve problems before they ever get to SPD.
There are many more benefits, but the more objective information a manager has about their department, the better they can advocate for and improve their department.
Ease of input and data complexity
Even if your team is on board with following the procedures to gather data about your department, it may not get done. Choose metrics that are easy to collect and analyze. Even though metric A  might be valuable to have, the complexity of collecting it could make it difficult to reliably obtain. However, with a little creativity, you might be able to collect metrics B and C and get the same insights you would have gotten from A.
might be valuable to have, the complexity of collecting it could make it difficult to reliably obtain. However, with a little creativity, you might be able to collect metrics B and C and get the same insights you would have gotten from A.
Inputting information is another hurdle worth accounting for. Rather than making your team type information into a system, incorporate preset functions and options that can be selected with one click. Better yet, incorporating barcodes and scanners removes many barriers to tracking.
Easy and accurate is better than difficult and perfect.
Explore what your tracking system can do
Many of our VOC members indicated they’ve only tapped into the potential features of their integrated tracking systems, and that there were opportunities to improve.
Here are a few tips for getting more out of your tracking system:
- Train, train, train. Most tracking solutions have training programs. Make sure that your team knows how to use your tracking system.
- Become friends with the administrator of your tracking system. Every facility administers software differently, and many times the tracking software used by SPD isn’t controlled by SPD at all. If this is the case, learn who manages the software and explore what they’re able to do with it. There may be functions available to you that you are not yet aware of.
- Use it. While there may be many features that aren’t enabled or configured for use, it’s important to use all of the ones available to you and understand their capabilities. Since you already own it, make the most of its capabilities.
Taking action
You’ve taken all the steps needed to start collecting data, but how long should you wait before you start taking action based on what you learn from it? The short answer: one month.
While you can wait a little longer, maybe 2-3 months, the fact of the matter is that if you start seeing opportunities to improve based on the data, it’s better for the department, the hospital, and most importantly, the patient to start improving as soon as you’re able to identify and solve the problem.
“To the SPD community: Don’t be afraid of data; data can be your best friend. It can be used to really positively impact your team and department.”
– Jhmeid Billingslea
With the tools and knowledge to start collecting data in your sterile processing department, it’s time to take the next step: using data to improve your department.
Check out the next blog in this series: Optimizing Your Department with Data
About: Voice of the Customer Committee
The Voice of the Customer Committee is a panel of healthcare and instrument reprocessing professionals who have graciously donated their time to share their expertise and guidance on current challenges faced by the instrument reprocessing community. Through sharing their insights, experiences, and best practices, we have been given the opportunity to share these findings with our readership. We’d like to thank our VOC members for their outstanding input and insights, as well as their time! Thank you for your continued partnership, and all you do.




 department.
department.

 automated flushing systems ensure that the appropriate volume of solution is flushed per device IFUs, meaning compliance comes at a much lower cost in time spent and repetitive motion.
automated flushing systems ensure that the appropriate volume of solution is flushed per device IFUs, meaning compliance comes at a much lower cost in time spent and repetitive motion.

 conversations: data.
conversations: data.
 adjustable sinks to accommodate employees of different heights and reduce the risk of workplace injuries. However, the budget of many departments don’t often allow for immediate upgrades to height-adjusted sinks. In those cases, a more affordable and practical solution can be a is a
adjustable sinks to accommodate employees of different heights and reduce the risk of workplace injuries. However, the budget of many departments don’t often allow for immediate upgrades to height-adjusted sinks. In those cases, a more affordable and practical solution can be a is a  such as carpal tunnel syndrome and other musculoskeletal disorders. To address this risk,
such as carpal tunnel syndrome and other musculoskeletal disorders. To address this risk,  and cleaning instruments. This requires optimal lighting that illuminates the working area and provides a clear view of the instruments. Many sterile processing and gastroenterology departments struggle with optimal lighting, which has a direct impact on eye strain.
and cleaning instruments. This requires optimal lighting that illuminates the working area and provides a clear view of the instruments. Many sterile processing and gastroenterology departments struggle with optimal lighting, which has a direct impact on eye strain. optimal height for each individual. However, with the use of
optimal height for each individual. However, with the use of  positions. Staff members may be hunched over sinks or prep and pack tables while manually cleaning or reaching too far for tools or materials at a prep & pack table. Performing these types of movements frequently can lead to musculoskeletal disorders if sinks and tables are unable to accommodate the height of the users. Many sinks also have deep basins, which are not conducive to manual cleaning and can cause lower back, shoulder, and neck pain.
positions. Staff members may be hunched over sinks or prep and pack tables while manually cleaning or reaching too far for tools or materials at a prep & pack table. Performing these types of movements frequently can lead to musculoskeletal disorders if sinks and tables are unable to accommodate the height of the users. Many sinks also have deep basins, which are not conducive to manual cleaning and can cause lower back, shoulder, and neck pain.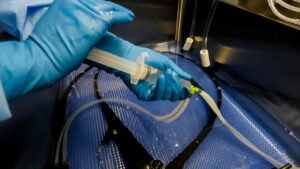 force, such as the manual cleaning of heavily soiled or narrow instruments, which can lead to strains and sprains. Repetitive motions, such as scrubbing instruments or using syringes when manually flushing, can lead to muscle fatigue and injuries such as carpal tunnel syndrome, which may result in missed shifts and staffing challenges.
force, such as the manual cleaning of heavily soiled or narrow instruments, which can lead to strains and sprains. Repetitive motions, such as scrubbing instruments or using syringes when manually flushing, can lead to muscle fatigue and injuries such as carpal tunnel syndrome, which may result in missed shifts and staffing challenges.





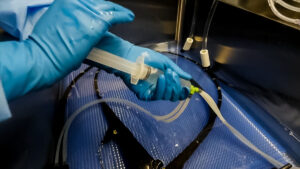 comes to no surprise, due to high turnover of both staff and scopes. With manual cleaning consuming a large chunk of time, use of automated flushing systems during the manual cleaning stage of endoscope reprocessing can result in more efficient and effective results compared to manual flushing. The devices built for automated flushing are designed to provide consistent flow and pressure during flushing. When two facilities used the Flush Buddy Systems, they found and increase in efficiency and consistent flush times.1
comes to no surprise, due to high turnover of both staff and scopes. With manual cleaning consuming a large chunk of time, use of automated flushing systems during the manual cleaning stage of endoscope reprocessing can result in more efficient and effective results compared to manual flushing. The devices built for automated flushing are designed to provide consistent flow and pressure during flushing. When two facilities used the Flush Buddy Systems, they found and increase in efficiency and consistent flush times.1  flushing with a syringe. According to
flushing with a syringe. According to