As we highlighted in a previous post, Workstation Disorganization: Problems and Opportunities, a myriad of problems can stem from a disorganized workstation. While it may seem like there isn’t enough space to organize your workstations adequately, there is a way: elevate your workstation.
Free up table space
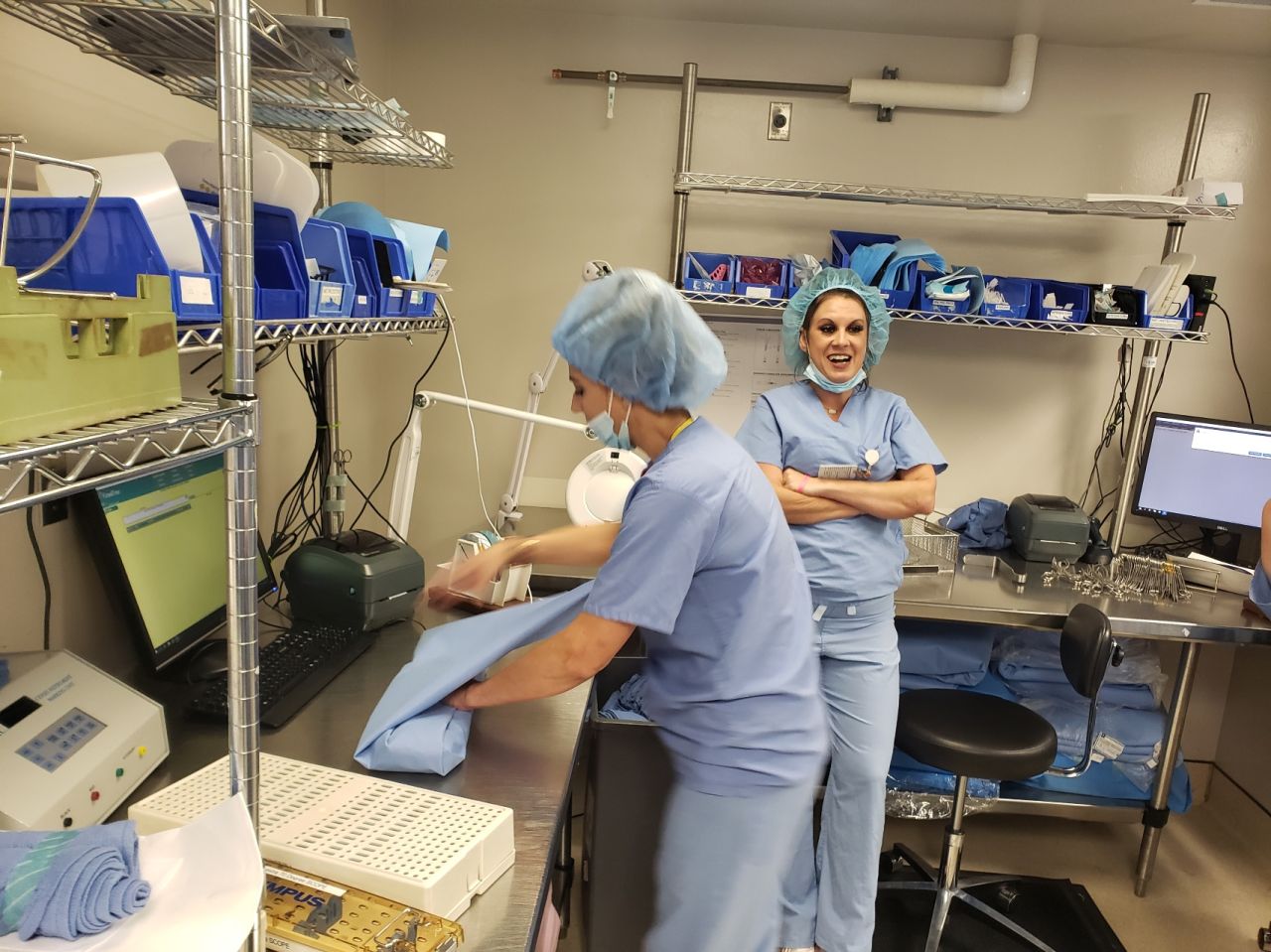
When analyzing a workstation and improving its configuration, one glaring problem is likely to come up before anything else: there isn’t enough space on the table surface for everything I need. It’s time to go above the tabletop and start utilizing vertical space.
The space above the back of most tables is generally under-utilized, if not entirely ignored. This vertical space is valuable real estate that can serve a multitude of purposes, including:
- Affixing storage bins for various consumables
- Mounting equipment, such as magnifiers
- Organizing computer and tracking systems
- Adding shelf space
- Add storage for blue wrap
Storage bins for consumables
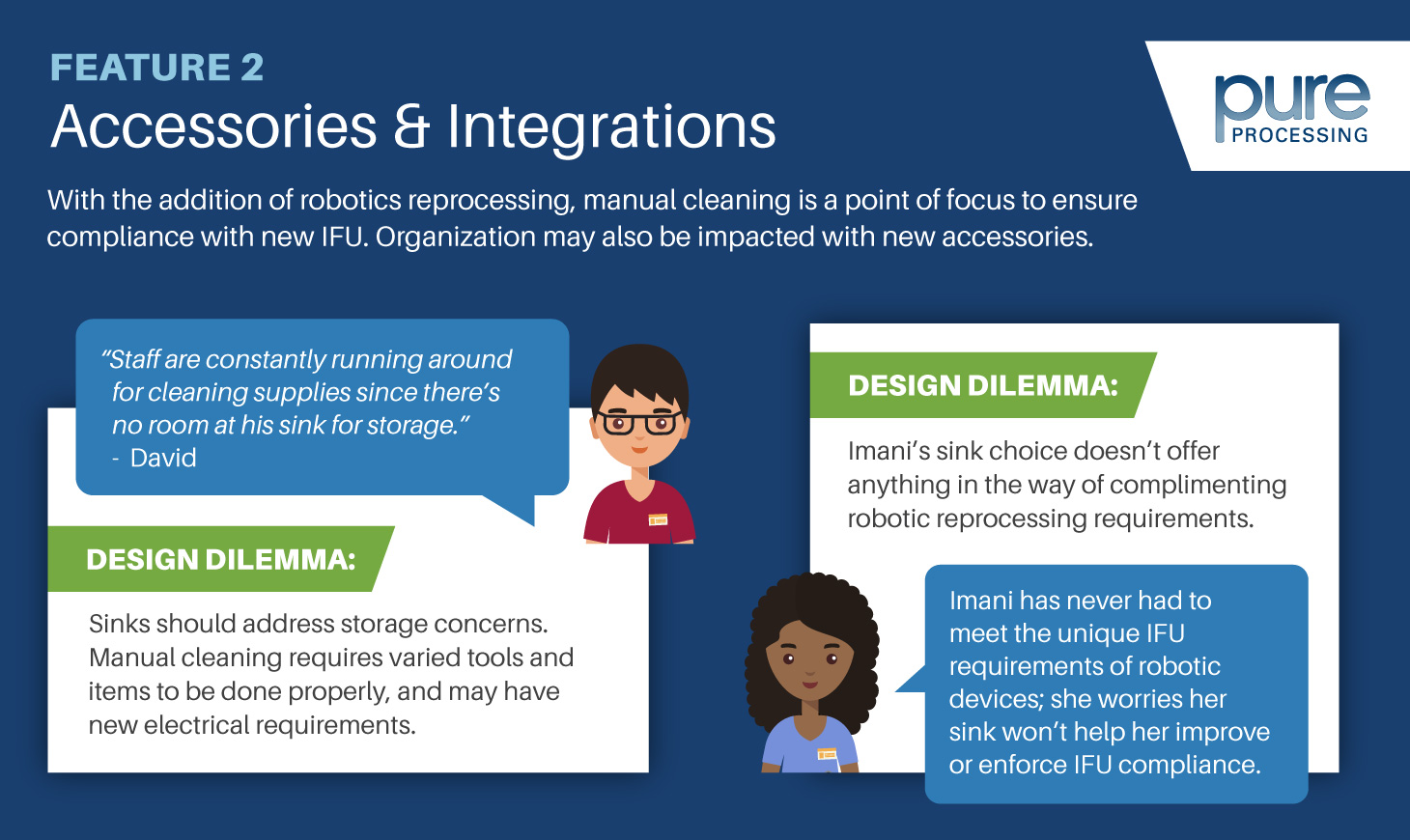
Many consumables and materials are required in an SPD department. Making sure that they’re all available and easily accessible at a workstation is a key to success for technicians.
Mounted storage bins enable custom configurations to allow for organization that facilitates streamlined workflows and faster packaging and assembly.
Mounted equipment
The tools used in SPD are critical to departmental success and, more  importantly, patient safety. All too often tools, such as magnifiers, are kept somewhere other than workstations, or deliberately moved from workstations because they inhibit technicians when not in use.
importantly, patient safety. All too often tools, such as magnifiers, are kept somewhere other than workstations, or deliberately moved from workstations because they inhibit technicians when not in use.
Mounting these tools are a game changer, and deliver three notable advantages:
- Tools can be maneuvered into technician’s area of activity when needed and shifted away when usage is complete. Mounted equipment is at the ready, but out of the way.
- Because the equipment is securely mounted to the back panel of the table, the risk of damage from falling or being knocked off of the table is mitigated.
- Mounted equipment doesn’t take up any valuable real estate on the surface of the workspace. What’s more, any power or data cords can be run behind the unit, reducing clutter and freeing up more working space.
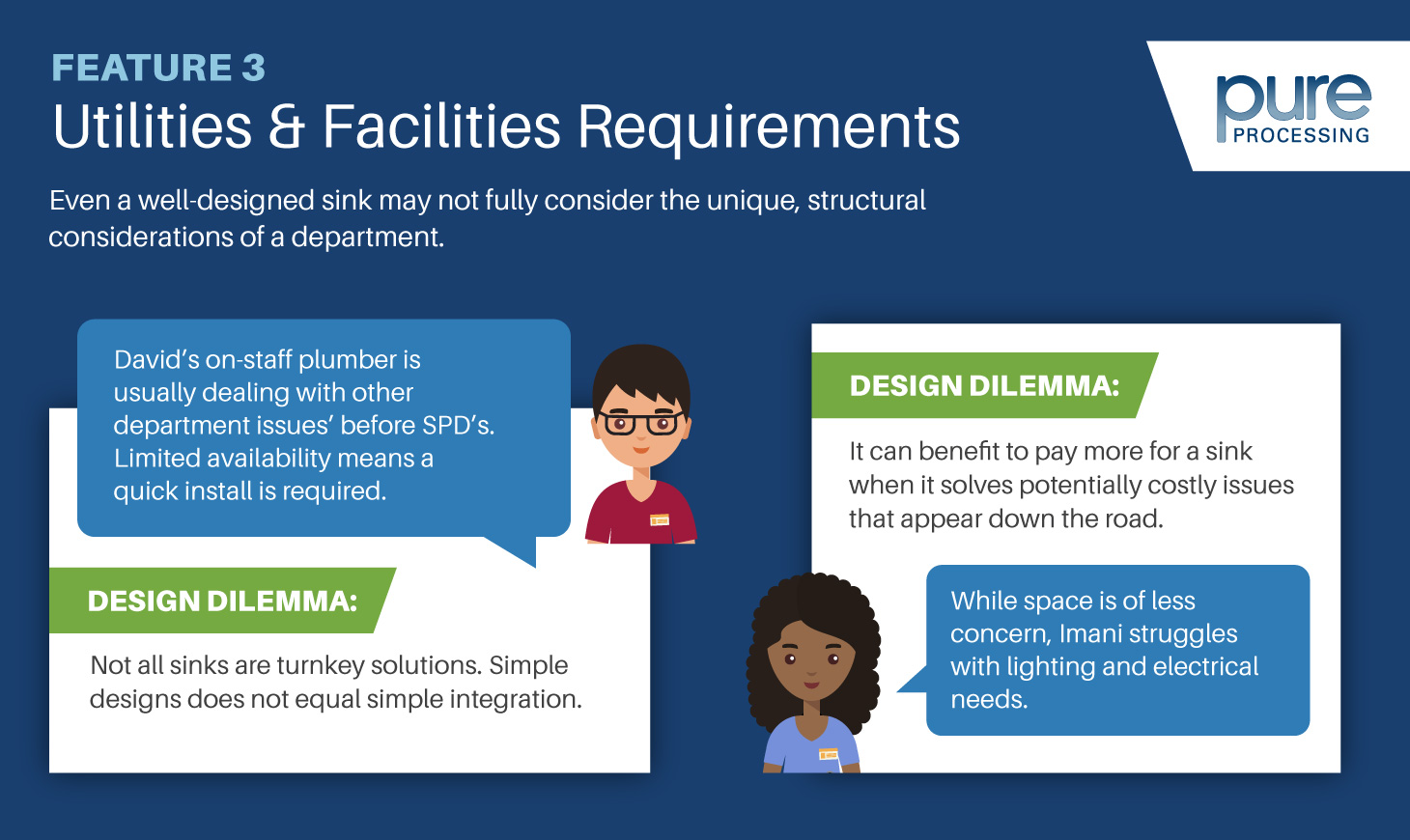
As new SPD-focused technology and equipment come out, a pegboard-style back wall will guarantee that your technicians have the space to make sure whatever they need is right at their fingertips.
Racks for blue wrap
 All too often, departments centralize blue wrap where space allows. Its also common that the central location isn’t located where wrapping takes place.
All too often, departments centralize blue wrap where space allows. Its also common that the central location isn’t located where wrapping takes place.
By relocating disposable blue wrap to its point-of-use, technicians spend noticeably less time preparing their tables for use.
Beyond the organizational benefits, having material elevated off of the workstation means that tools, such as built-in tabletop lighting, can be better utilized, ensuring that every wrap is suitable for the sterilization process.
Elevate (and adjust) your Workspace!
Getting equipment and materials ‘elevated’ to vertical space along a back wall is tremendously beneficial in terms of organization, effectiveness, and efficiency. But there’s another variety of elevation that can have a big impact on the work done at a workstation: ergonomics!
The ability to elevate – and lower – a workstation’s height can improve the comfort and efficiency for technicians. A fixed-height workstation might be good for one team member’s ergonomic ‘Goldilocks’ zone, but for all others, that table is less compliant. A couple benefits of height-adjustable tables include:
- Effective use of the table’s organization and layout. When mounted accessories can also change height with the table, the entire station is optimized for ergonomic benefit.
- When technicians need to reach or stoop at their workstation, it can cause strain or injury over time. By offering an ergonomic solution at each workstation, instances of strain and injury can be substantially reduced.
Have a problem or challenge you’d like to tackle? Let us know!




 they can get in the way if they are not given a deliberate, permanent home. Assembling a rigid container becomes difficult with a keyboard in the way. Inspecting an instrument is also challenging when a monitor blocks the magnifier.
they can get in the way if they are not given a deliberate, permanent home. Assembling a rigid container becomes difficult with a keyboard in the way. Inspecting an instrument is also challenging when a monitor blocks the magnifier. Inspecting and packaging requires immense attention to detail; not only does everything need to be executed flawlessly to ensure patient safety, but the correct materials and equipment need be employed based on the types of instruments and the sterilization processes they require. A variety of small issues can arise.
Inspecting and packaging requires immense attention to detail; not only does everything need to be executed flawlessly to ensure patient safety, but the correct materials and equipment need be employed based on the types of instruments and the sterilization processes they require. A variety of small issues can arise. than just divert their focus; it can slow them down considerably. A good process produces good results, and good processes require organization. After all, it was an industrial level of organization that made Henry Ford’s production line so successful.
than just divert their focus; it can slow them down considerably. A good process produces good results, and good processes require organization. After all, it was an industrial level of organization that made Henry Ford’s production line so successful. Let us briefly revisit the meal you are preparing to impress your spouse. The first thing you would probably do is gather your ingredients. You head to the fridge for a protein and vegetables, then to the pantry for some pasta, and finally grab a few spices from the cabinet.
Let us briefly revisit the meal you are preparing to impress your spouse. The first thing you would probably do is gather your ingredients. You head to the fridge for a protein and vegetables, then to the pantry for some pasta, and finally grab a few spices from the cabinet.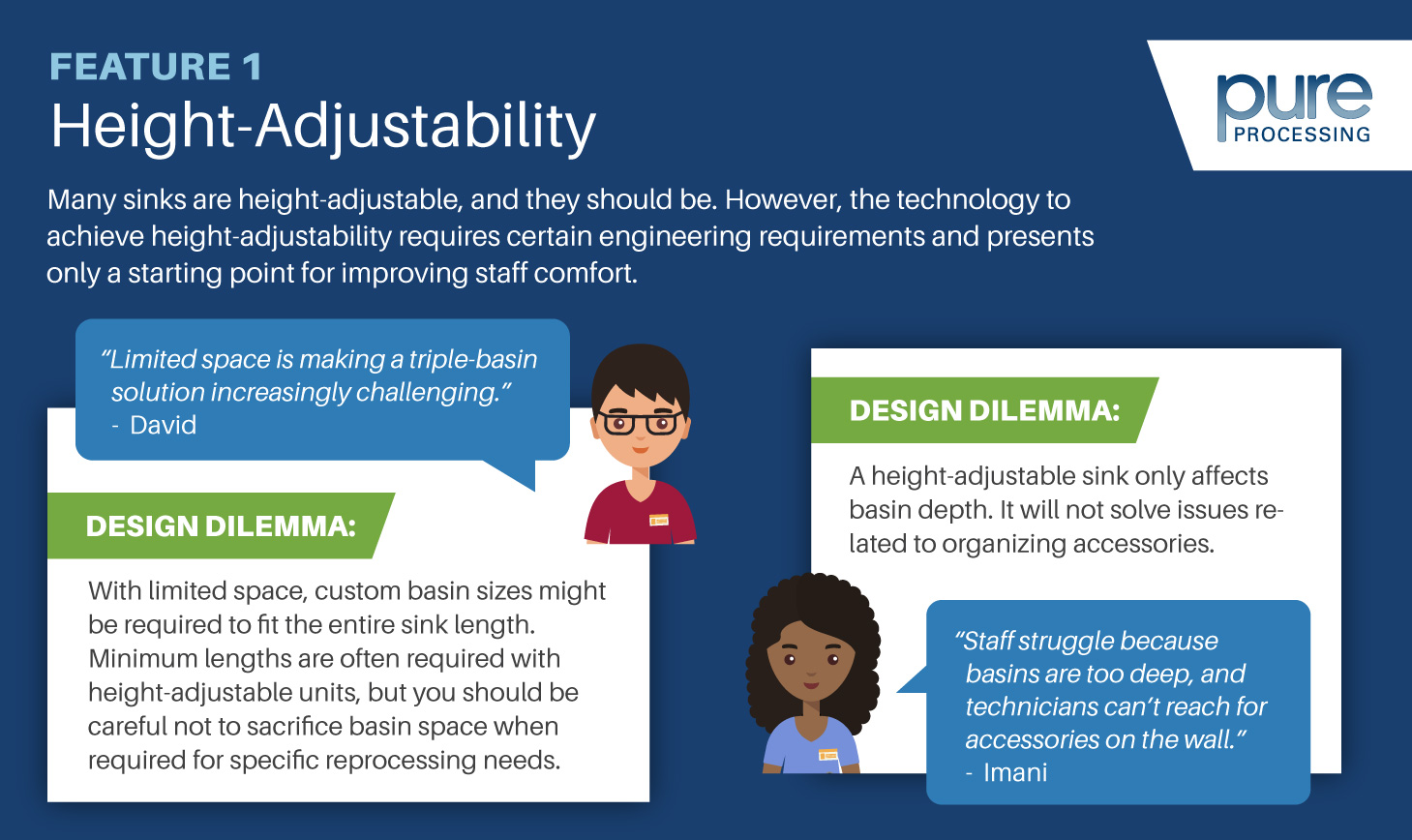
 critical water for ultrasonic rinsing.
critical water for ultrasonic rinsing. et your teammates involved: Next level leadership, infection control, educator (SPD or OR), and a facility Joint Commission readiness team allows your team to holistically engage on behalf of patients, and able to see a 360-degree engaged team view for solving quality problems. Teammates can be of great assistance in training, audits, and keeping SPD updated with regulatory requirements.
et your teammates involved: Next level leadership, infection control, educator (SPD or OR), and a facility Joint Commission readiness team allows your team to holistically engage on behalf of patients, and able to see a 360-degree engaged team view for solving quality problems. Teammates can be of great assistance in training, audits, and keeping SPD updated with regulatory requirements. One set at a time: Take a basic, frequently used set, and verify you have all the reference numbers. Standardize the products in these sets and then move on to the next. Use your primary instrument vendor for data and on-site assistance. Utilize their services for maintaining stock, most-used items, and assisting with pegboard creation and maintenance.
One set at a time: Take a basic, frequently used set, and verify you have all the reference numbers. Standardize the products in these sets and then move on to the next. Use your primary instrument vendor for data and on-site assistance. Utilize their services for maintaining stock, most-used items, and assisting with pegboard creation and maintenance.
 SPD leaders can show industry recommendations to gain additional full time staff support. Time studies regarding the actual safe handling and inspection of instruments with reference to the IFU need to be published to back that need up. Without that information, a question arises: If we could do it with three people before, why do we need six? The fact is, no one wants to admit that processes have not been done correctly, completely, or safely in the past. Quality product was often more a result of luck than best practice or MFC adherence. Most departments don’t have the time, people power, or wherewithal to perform their own holistic time and motion studies to validate. Time and again we return to “what we have been doing is good enough.” Only recently has the “people factor” in the work environment been brought to light on our most important assets, thanks to the AAMI focus on maintaining safe body temperatures, more frequent breaks, and hydration necessities.
SPD leaders can show industry recommendations to gain additional full time staff support. Time studies regarding the actual safe handling and inspection of instruments with reference to the IFU need to be published to back that need up. Without that information, a question arises: If we could do it with three people before, why do we need six? The fact is, no one wants to admit that processes have not been done correctly, completely, or safely in the past. Quality product was often more a result of luck than best practice or MFC adherence. Most departments don’t have the time, people power, or wherewithal to perform their own holistic time and motion studies to validate. Time and again we return to “what we have been doing is good enough.” Only recently has the “people factor” in the work environment been brought to light on our most important assets, thanks to the AAMI focus on maintaining safe body temperatures, more frequent breaks, and hydration necessities. employee satisfaction to maintained standard compliance. It is critical to consider when adding new equipment. You’ll want to look at your caseload, your specialties, and how many technicians you have.
employee satisfaction to maintained standard compliance. It is critical to consider when adding new equipment. You’ll want to look at your caseload, your specialties, and how many technicians you have.




