Assembly teams carry a huge responsibility: they ensure instrumentation is functional, packs are accurately assembled, and everything is neat and orderly for their operating room staff.
The humble prep and pack table may be easily forgotten in this mission. At best, it does an adequate enough job as a place to assemble trays and packs. At worst, a prep and pack table actively reduces the quality and quantity of work an SPD can provide.
There are three common reasons assembly tables may be failing your team, your department’s objectives, and reducing your department’s potential.
1: Your sterile processing workstations are reducing your productivity
Every sterile processing department is heavily scrutinized for the quality and quantity of its work. The prep and pack table is not always considered crucial to this equation; a table is a table, after all. But, as one SPD Manager describes it:
“Why am I expected to deliver high end outcomes for my Patients and Hospital, but being asked to do this with low-end, mediocre technology for my Staff?”

A good starting point to assess current tables is to evaluate their actual workspace. As trays and instrumentation becomes larger, the tools required to assemble increase, and volume doubles or triples, every inch of workspace is valuable.
Actively cluttered and disorganized tables can cause mistakes; picking the wrong tool or instrument, missing a screw to assemble a device, or placing the wrong label on a tray can be a mistake that makes it way into the OR. An already scrutinized department can’t afford these avoidable mistakes.
As tables are left cluttered, the disorganization spreads elsewhere. As another SPD Manager describes their current workflow:
“The design of my current table hinders productive space, forcing our Techs to work in cramped spacing,
and to zig-zag through the department to complete a single tray. Without high-end lighting, and high-end production tools, it places at risk of QC assessments, and therefore, the Patient.”
SP technicians already lack the time they need to do their job. Further wasting minutes and hours leaving an assembly table to find tools or space to assemble is avoidable lost time.
At a high-level, consider even a conservative loss of time when technicians leave their assembly table for 2 minutes for every tray assembled:
| Hourly Wage for a Technician | $25/hour |
| Minute Wages for Same Tech | $0.42 |
| Time/Motion Lost per Tray (leaving an assembly table to finish a Tray) | 2 minutes lost |
| Trays Process on Avg per Day | 300 |
| # of Working Days Processing, per Year | 240 |
| Potential Lost Worker Time / Year | $60,480.00 |
If staff were observed to lose 5 minutes per tray because of productivity inefficiencies, the potential outcome is even more worrying:
| Hourly Wage for a Technician | $25/hour |
| Minute Wages for Same Tech | $0.42 |
| Time/Motion Lost per Tray (leaving an assembly table to finish a Tray) | 5 minutes lost |
| Trays Process on Avg per Day | 300 |
| # of Working Days Processing, per Year | 240 |
| Potential Lost Worker Time / Year | $151,200.00 |
Well-designed assembly tables can reduce, even conservatively, 2 minutes of time to access lights, heat sealers, scales, and other tools, saving departments thousands of worker dollars.
Finally, consider if your table vendor provides everything for a well-rounded assembly table. From common needs like task lights to integrated computers to often forgotten electrical support, a great table may fail the test without passing scores in other key areas.
Productivity is the first and often most important metric that decision makers will evaluate from sterile processing: consider if your prep and pack table is failing you in this area.
2: Your sterile processing workstations aren’t truly “ergonomic”
Ergonomics is more than comfort. A lack of poor ergonomics can lead to mistakes, costs, and at worst, non-compliance. Poor considerations of lighting at an assembly table can lead to missed mistakes, and compromised patient safety. A recent update to the AORN Guideline for Manual High-level Disinfection directly addresses ergonomics as a key factor to improving patient outcomes1:
“Staff discomfort can lead to mistakes and non-adherence to processing guidelines. AORN recommends the sterile processing area have ergonomic features to reduce staff discomfort. Ergonomic features can include work surfaces and sinks at a comfortable height. Also, there should be space to perform cleaning with sufficient lighting.”
From the tiniest ergonomic details like footrests and the storage of heavy items, to more significant factors like adequate lighting as described in ST79 and ST91, lack of ergonomic considerations leads to mistakes and non-compliance.

Some might argue that their prep and pack tables are height-adjustable, but are they really? Hand-cranked height-adjustable tables cover up one deficiency with another. And, if the table set-up cannot also raise and lower with the workspace, then the prep and pack table doesn’t consider the entire worker’s space. Staff may find themselves excessively bending, hunching, reaching, or straining to use tools and computers.
Beyond these factors, poor ergonomics plainly leads to an increased risk of worker’s compensation claims:
“We’ve had 2400 lost worker hours this year due to various injuries, and a turnover of (8) employees, of whom (4) were assigned to our Clean Side due to poor ergonomics, and cramped working conditions.”
3: Your sterile processing workstations aren’t lasting
When your SPD is finally bestowed resources to upgrade and enhance, the opportunity cannot be wasted on poor investments. Prep and pack table projects can be costly; replacing up to 10, 12 or more stations is a major investment of both time and cost.
If an assembly table cannot be readily serviced or is frequently experiencing issues, it may be failing you. Even tables can come with unforeseen costs, as one Manager describes:
“My current Tables have lasted 8 Years, complete with service issues. They are not enabling my Team to be more productive, while protecting them.”
An investment into tables comes with a commitment; they may be in place for over 10 years. Plan for the future. If the assembly table cannot grow with the needs of your team or the direction of your hospital and system, it may not be the right choice. A glance at your current tables might reveal a patchwork of band-aid solutions meant to make a static investment grow with your needs.
Sterile processing bears a lot of demands. Patients cannot afford any mistakes in reprocessing. Administration cannot afford lost worker hours, workers compensation costs, and poor investments. As a department leader, you cannot afford to fight with your equipment. Careful evaluation of even the humblest piece of equipment, the prep and pack table, might reveal hidden failures affecting all of these areas. With these failures identified, they can now reveal focuses for future projects.
Exploring options to replace your existing prep and pack tables? Continue reading on to identify the qualities in a table that enable productivity, boost ergonomics, and deliver long-term ROI!
Works Cited









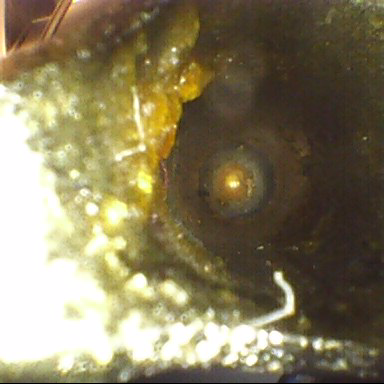
 test results.
test results.
 protein-based bioburden cleaned in sterile processing and GI. Collagen is also a protein, which makes up the body’s skin, tones, tendons, and ligaments. Protein is a major structural component in the body made up of folded chains of amino acids and make up our cells and tissues, as well as many enzymes and hormones.
protein-based bioburden cleaned in sterile processing and GI. Collagen is also a protein, which makes up the body’s skin, tones, tendons, and ligaments. Protein is a major structural component in the body made up of folded chains of amino acids and make up our cells and tissues, as well as many enzymes and hormones.
 difficult to clean, so then must the technology evolve to clean them.
difficult to clean, so then must the technology evolve to clean them.